Revista Científica UDO Agrícola Volumen 9.
Número 4. Año 2009. Páginas: 972-978
Bioconversion of maize husk into value added ruminant feed by using
white-rot fungus
Bioconversión de la tusa de maíz como valor agregado en la alimentación de ruminates mediante el uso de hongos de la pudrición blanca
Abayomi
AKINFEMI ![]() 1, Olaniyi
Jacob BABAYEMI 2 and Segun Gbolagade JONATHAN 3
1, Olaniyi
Jacob BABAYEMI 2 and Segun Gbolagade JONATHAN 3
1Nasarawa State
University, Keffi, Faculty of Agriculture, Department
of Animal Science, PMB 135, Shabu-Lafia, Nigeria; 2Department
of Animal Science, University of Ibadan, Nigeria and 3Department of
Botany and Microbiology, University of Ibadan, Nigeria. Email: akinfemiabayomi2003@yahoo.com ![]() Corresponding author
Corresponding author
|
Received: 07/30/2009 |
First reviewing ending:
10/05/2009 |
|
First review received: 11/15/2009 |
Accepted: 11/29/2009 |
ABSTRACT
Treatment
of crop wastes with some species of white-rot fungi can enhance the nutritive
value. After solid state fermentation of maize (Zea mays L.) husk MH with four white-rot fungi (Pleurotus tuber-regium, Pleurotus
pulmonarius, Pleurotus sajor caju and Lentinus subnudus) for 40 days, the chemical composition and in vitro digestibility of the resulting
substrates was evaluated. Biodegradation increased the crude protein from 7.44%
for untreated MH, (UM, control) to 9.89% for L. subnudus (LSM); 9.67% for P. tuber-regium (PTM)
and 9.90% for P. pulmonarius
(PPM) and 9.55% for P. sajor caju (PSM). In
contrast, growth of fungi reduced crude fiber (CF) from 30.45% (UM) to 22.27,
24.29, 19.07 and 14.14% for LSM, PTM, PPM and PSM, respectively. The preference
for cellulose and hemicellulose utilization by the fungi was indicated by
decrease in values obtained. LSM had the least value for cellulose (27.43 %)
while PSM with a hemicellulose content of 18.46% recorded the highest reduction
in hemicellulose. There were significant differences (p ≤ 0.05) between
the treated and untreated maize husk in terms of metabolisable
energy (ME), organic matter digestibility (OMD) and short chain fatty acid
(SCFA) as measured by the in vitro
gas production method using ruminal microflora. OMD ranged from 38.28-48.97%
with highest values for fungal treated husk and lowest for untreated substrate.
LSM showed the highest (p ≤ 0.05) values for SCFA and ME for all the
substrates under study. All the fungal treated substrates had the highest in vitro fermentation characteristics
and cumulative gas production. Result of this study show that fungal treatment
of maize husk enhanced digestibility by increasing the crude protein and
decreasing the crude fiber.
Key words: Maize husk,
white-rot fungi, biodegradation, in vitro
digestibility.
RESUMEN
El tratamiento de residuos de cosechas con algunas
especies de hongos causantes de la pudrición blanca puede mejorar su valor
nutritivo. Después de la fermentación en estado sólido de tusas de maíz (TM) (Zea mayz L.)
con cuatro hongos causantes de la pudrición blanca (Pleurotus tuber-regium, Pleurotus pulmonarius, Pleurotus sajor caju y Lentinus subnudus)
durante 40 días se evaluó la composición química y digestibilidad in vitro de los sustratos resultantes. La
biodegradación incrementó la proteína cruda de 7,44% para las TM no tratadas
(TMNT, control) a 9,89% para L. subnudus (TMTLS); 9,67% para P. tubérculo-regium (TMTPT); 9,90% para P. pulmonarius
(TMTPP) y 9,55% para P. sajor caju (TMTPS). Por el
contrario, el crecimiento de los hongos redujo la fibra cruda de 30,45% (TMNT)
a 22,27; 24,29; 19,07 y 14,14% para TMTLS, TMTPT, TMTPP y TMTPS, respectively. La preferencia por la utilizacion
de celulosa y hemicelulosa de los hongos fue indicada
por la disminución en los valores obtenidos. TMTLS (27,43%) tuvo el menor valor
para la celulosa, mientras que TMTPS (18,46%) registró la menor reducción de la
hemicelulosa. Hubo diferencias significativas (p ≤
0,05) entre las TM tratadas y no tratadas en términos de energía metabolisable (EM), digestibilidad de la materia orgánica
(DMO) y de ácidos grasos de cadena corta (AGCC). La DMO varió entre
38,28-48,97% y los mayores valores fueron aquellos de las TM tratadas con
hongos y los menores para las no tratadas. TMTLS mostró el valor más alto (p≤0,05)
para AGCC y EM para todos los sustratos en estudio. Todos los sustratos
tratados con hongos tuvieron los mayores valores para las características de
fermentación in vitro y producción
acumulada de gas. Los resultados de este estudio mostraron que el tratamiento
de TM con hongos mejoró la digestibilidad mediante el incremento de la proteína
cruda y la disminución de la fibra cruda.
Palabras clave: Tusa de maíz, hongos causantes de la pudrición
blanca, biodegradación, digestibilidad in
vitro.
INTRODUCTION
In Nigeria,
ruminant animal suffer from under feeding especially during the dry seasons due
to shortage of forages and more so because of low nutritive value of available
crop wastes. The available wastes such as maize stover,
corn cob, and maize husk cereal straws are not able to meet the nutritional
requirements of ruminants. To dispose these wastes, they are usually burnt in
heaps thereby releasing offensive odor and gases into the atmosphere. Some are
even thrown into the rivers and streams thereby endangering aquatic life
(Jonathan et al, 2008). Maize husks
are fibrous leafy materials covering the maize ear. They are usually heaped in
refuse dump, farm lands and sometimes near homes. They usually constitute
nuisance to the environment and are rarely relished by ruminant animals because
of their tough nature and low nutritive value.
Maize husk in spite
of its limitations could be recycled and used as a source of valuable lignocellulosics biomass for animals if treated with fungi.
Belewu and Okhawere (1998),
reported on the delignification and nutritive values of rice husk and sorghum stover treated with Trichoderma harzanium. They observed increase in crude protein
content and decrease in crude fiber content of the fungal treated substrates.
Cultivation of edible mushrooms like Pleurotus tuber-regium, Pleurotus pulmonarius, Pleurotus sajor caju and Lentinus subnudus on lignocellulosic wastes may thus be valuable for converting
these materials, which are considered to be waste into protein rich ruminant
feeds. The cultivation and harvest of these fungi on maize husk, apart from
enriching the substrate, may also offer economic incentive for agribusiness. In
view of the paucity of information on this subject, it is therefore necessary
to examine the influence of cultivating edible mushrooms on the chemical
composition and in vitro fermentation
of maize husk. This study was therefore conducted to provide information on the
possibility of converting maize husk into a value added feedstuff for ruminant
feed via solid state fermentation.
MATERIALS
AND METHODS
Sample
Collection
Dried samples of
maize residues (maize husk) and were collected from the Teaching and Research
Farm, University of Ibadan, Ibadan, Nigeria. The materials were milled and
oven-treated at 650C until a constant weight was obtained for any
dry matter determination.
The
fungus
The sporophores of Pleurotus tuber-regium, Pleurotus pulmonarius, Pleurotus sajor caju and Lentinus subnudus growing in the wild were collected from Ibadan
University botanical garden. These were tissue cultured to obtain fungal
mycelia (Jonathan and Fasidi, 2001). The pure culture
obtained was maintained on plate of potato dextrose agar.
Degradation
of maize husk by P. tuber-regium, P. pulmonarius,
P. sajor caju and L. subnudus
Preparation of substrate
Jam bottles used
for this study were thoroughly washed and dried for 10 min at 100oC.
Twenty five grams of the dried milled substrate was weighed into each jam
bottle and 70ml distilled water was added. The bottles were immediately covered
with aluminum foil and sterilized in the autoclave at 121oC for 15
min .Each treatment was conducted in triplicate.
Inoculation
Each bottle was
inoculated at the center of the substrate with two, 10.00 mm mycelia disc and
covered immediately. They were kept in a dark cupboard in the laboratory at 300C
and 100% relative humidity. After 40 days of inoculation, the experimental
bottles were harvested by autoclaving again to terminate the mycelia growth.
Samples of the biodegraded samples were oven dried to constant weight for
chemical analysis and in vitro
digestibility.
In
vitro gas production
Rumen fluid was obtained
from three West African Dwarf female goats through a suction tube before the
morning feed. The animals were fed with 40% concentrate feed (40% corn, 10%
wheat offal, 10% palm kernel cake, 20% groundnut cake, 5% soybean meal, 10%
brewers grain, 1% common salt, 3.75% oyster shell and 0.25% fishmeal) and 60%
Guinea grass. Incubation was carried out according to Menke
and Steingass (1998) in 120ml calibrated syringes in
three batches at 390C. To 200mg sample in the syringe was added 30ml
inoculum containing cheese cloth strained rumen liquor and buffer (9.8g NaHCO3 + 2.77g Na2HPO4
+ 0.57g KCL + 0.47g NaCl + 0.12g MgSO4. 7H20
+ 0.16g CaCI2 . 2H20 at a ratio of 1:4 v/v under
continuous flushing with CO2. The gas production was measured at 3, 6, 9,
12, 15, 18, 21 and 24h. After 24 hours of incubation, 4ml of NaOH (10M) was introduced to estimate the amount of methane
produced (Fievez et
al., 2005). The average volume of gas produced from the blanks was deducted
from the volume of gas produced per sample. The gas production characteristics
were estimated using the equation Y = a + b (1-ect) described by Ǿrskov and McDonald (1979), where Y = volume of gas
produced at time‘t’ a = intercept (gas produced from
the soluble fraction, b = gas production from the insoluble fraction, a+b= final gas produced, c = gas production rate constant
for the insoluble fraction (b), t = incubation time. The post incubation
parameters such as metabolisable energy (ME, MJ/kg
dry matter (DM)) and organic matter digestibility (OMD %) and short chain fatty
acids (SCFA) were estimated at 24h post gas collection according to Menke and Steingass, (1988).
ME = 2.20 + 0.136* Gv +
0.057* CP + 0.0029*CF
OMD = 14.88 +
0.88Gv + 0.45CP+ 0.651XA
SCFA = 0.0239*Gv - 0.0601;
Where
Gv, CP, CF and XA are net gas production (ml/200mg,
DM), crude protein, crude fiber and ash of the incubated sample, respectively.
Chemical composition
DM was determined
by oven drying the milled samples to a constant weight at 1050C for
8 hours. Crude protein was determined as Kjeldhal
nitrogen x 6.25. Ether extracts, crude fiber and ash was
determined according to (AOAC, 1995) method. Neutral detergent fiber (NDF),
acid detergent fiber (ADF) and acid detergent lignin (ADL) was determined using
the method described by Van Soest et al (1991). Hemicellulose was
calculated as the difference between NDF and ADF while cellulose is the
difference between ADF and ADL.
Statistical
analysis
Data obtained were
subjected to analysis of variance (ANOVA).Where significant differences
occurred, the means were separated using Duncan multiple range F-test of SAS
(Statistical Analysis System Institute Inc., 1998) option.
RESULTS AND DISCUSSION
Chemical composition
Shown in Table 1 are
the results of chemical composition (g/100g DM) of maize husk treated with four
different fungi. A wide variation exists in the different results obtained. The
results show a significant (p ≤ 0.05) increase in the crude protein (CP),
ether extract (EE) and ash contents after the fungal treatment. The CP
increased from 7.44% (UM) to 9.90% (PPM). The improved CP value obtained in the
fungal treated maize husk could be due to the release of polysaccharide bound
protein and this makes the substrate nutritionally better (Belewu
et al, 2003). This agrees with the
report of Broerse and Visser
(1996) who stated that the extra cellular enzymes secreted by
the fungus contains amorphous homo and heteropolysaccharides
which often in association with protein. Rice husk treated with Trichoderma harzanium
recorded a similar increase in nutrient composition and this has been found to
compensate for the low and poor protein content of concentrate diets of raw
straw and hay consumed by animals in the tropical environment (Belewu 2001; Belewu and Banjo,
1999). Crude fiber contents (NDF, ADF, ADL, cellulose and hemicellulose) which
were observed to consistently reduce in all the four fungi treated substrates
agrees with report of Belewu (2001). The decrease in
NDF concentration may be attributed mainly to the extensive utilization of
hemicellulose by fungi (Chen et al,
1995).The preferential degradation of cellulose and hemicellulose could be the
result of type of substrate, duration of degradation and physiological
behaviors of the fungi used.
|
Table
1. Chemical composition (g/100g DM) of degraded maize husk by four strains of
fungi. |
||||||
|
Parameters |
UM |
LSM |
PTM |
PSM |
PPM |
SEM |
|
Dry Matter |
88.85a |
86.78b |
86.89b |
86.44b |
87.70a |
0.00 |
|
Crude Protein |
7.44b |
9.89a |
9.67a |
9.55a |
9.90a |
0.30 |
|
Ether extract |
1.27b |
2.52a |
1.75a |
2.78a |
2.82a |
0.21 |
|
Ash |
3.32c |
3.53b |
3.90a |
3.86a |
10.37a |
0.26 |
|
Crude fiber |
30.45a |
22.27c |
24.29b |
14.15e |
19.07d |
0.00 |
|
NFE |
42.48a |
36.43b |
39.49ab |
39.49ab |
42.18a |
0.63 |
|
ADF |
49.15a |
39.27d |
41.05c |
44.09b |
43.94b |
0.30 |
|
NDF |
71.14a |
58.96c |
60.58c |
62.55b |
63.13b |
0.33 |
|
ADL |
14.87a |
11.54b |
11.89b |
11.27b |
12.14b |
0.28 |
|
Cellulose |
34.25a |
27.73d |
29.43c |
32.82b |
31.80b |
0.24 |
|
Hemicellulose |
21.99a |
19.69b |
19.53b |
18.46b |
19.90b |
0.33 |
|
Small
case letters imply means in the same row with different superscripts are
significantly varied (p ≤ 0.05). UM
= untreated maize husk (control), LSM = Lentinus subnudus degraded maize husk, PTM = Pleurotus tuber-regium
degraded maize husk, PSM = Pleurotus sajor caju degraded maize
husk, PPM = Pleurotus
pulmonarius degraded maize husk, SEM = Standard
error of the mean, NFE = Nitrogen Free Extract, ADF = acid detergent fibre,
NDF = neutral detergent fiber and ADL = acid detergent lignin. |
||||||
Gas volume
The in vitro gas production over the period of
24h is shown in Table 2. As could be seen from the trend displayed by the
treated substrates more gas production is still possible beyond 24h. There are
many factors that may determine the amount of gas produced during fermentation,
depending on the nature and level of fiber (Babayemi, et al., 2004) and potency of the rumen
liquor used for incubation (Babayemi, 2007). Generally, as cited by Babayemi
(2007) gas production is a function and mirror of degradable carbohydrate and
therefore, the amount of gas produced depends on nature of the carbohydrates (Demeyer and Van Nevel,1975; Bummel and Becker, 1997). All the four fungi used improved
gas production, an indication of better digestibility of the treated substrate.
Sommart et al.,
(2000) suggested that gas volume is a good parameter from which to predict
digestibility, fermentation end product and microbial protein synthesis of the
substrate by rumen microbes in the in
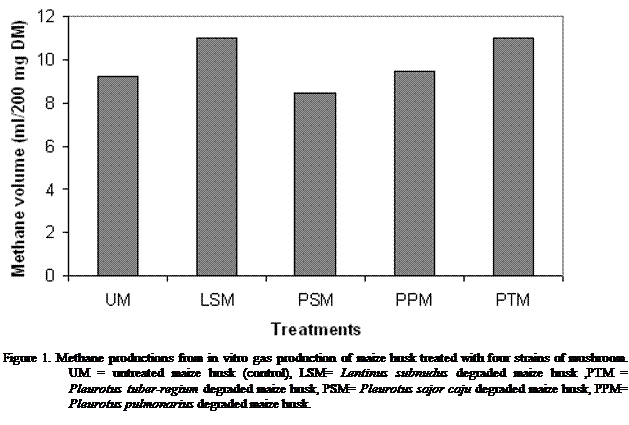
vitro system. Methane productions (Figure 1) were highest in the substrate
treated by LSM and PTM with the lowest methane production in PSM. Methane
production is an energy loss to the animal; this implies that there would be
the need for energy supplementation in the diet of the ruminant to replenish
this loss.
|
Table
2. In vitro gas production (ml/200
mg DM incubated) from maize husk degraded by four strains of mushroom for a
period of 24 hours. |
||||||||
|
|
Incubation period (h) |
|||||||
|
Treatments |
3 |
6 |
9 |
12 |
15 |
18 |
21 |
24 |
|
UM |
8.67b |
12.50b |
14.00b |
14.67b |
16.00b |
17.67b |
19.00b |
20.33d |
|
LSM |
13.00a |
16.33a |
17.67a |
19.00a |
20.30a |
21.67a |
22.67a |
29.00a |
|
PTM |
12.00a |
15.00a |
17.67a |
19.33b |
19.67a |
22.00a |
22.67a |
23.67c |
|
PSM |
12.50a |
16.00a |
18.33a |
19.33b |
20.33a |
21.33a |
22.00a |
26.33b |
|
PPM |
12.50a |
14.67a |
18.00a |
19.33b |
20.33a |
20.67ab |
22.67a |
26.00b |
|
SEM |
0.32 |
0.56 |
0.28 |
0.40 |
0.40 |
0.64 |
0.90 |
1.20 |
|
Small
case letters imply means in the same column with different superscripts are significantly
varied (p ≤ 0.05), UM = untreated maize husk (control), LSM= Lentinus subnudus
degraded maize husk ,PTM = Pleurotus tuber-regium degraded maize husk, PSM= Pleurotus sajor caju
degraded maize husk ,PPM= Pleurotus pulmonarius
degraded maize husk and SEM = Standard error of the mean |
||||||||
Gas
production characteristics
Gas production from
the fermentation of treated and untreated maize husk was measured at 3, 6, 9,
12, 15, 18, 21 and 24 hours using in
vitro gas production technique. The results are presented in Table 3. The
gas volumes at asymptote (b) describe the fermentation of the insoluble but
degradable fraction. It can be seen from the result obtained from this study
that value obtained for (b) in the treated maize husk was higher than the
untreated, possibly a reflection of the decreased CF and ADL. Furthermore, the
fungi used enhanced the CP of the treated substrates. Getachew
et al., (1999) reported that gas
production is basically the result of fermentation of carbohydrate into
acetate, propionate and butyrate. The high fermentation obtained for all the
fungi treated maize husk may possibly be influenced by the carbohydrate
fractions readily available to the microbial population (Chumpawadee
et al.2007). The fast rate of gas
production (c) obtained in the PTM treated maize husk and the untreated
substrate (UM) could mean that the carbohydrate were readily available to rumen
microbial population. Slower rates were however obtained in LSM, PSM and PPM
may be due to specie differences of the fungi used.
|
Table 3. Estimated gas production characteristics,
estimated organic matter digestibility (OMD), short chain fatty acid (SCFA)
and metabolisable energy (ME). |
||||||
|
Parameters |
UM |
LSM |
PTM |
PSM |
PPM |
SEM |
|
Gas production characteristics |
||||||
|
(a+b) (ml) |
20.33d |
29.00a |
23.67c |
26.33b |
26.00b |
0.33 |
|
b (ml) |
16.66e |
16.00a |
11.67d |
13.83b |
13.50c |
0.02 |
|
c (h-1) |
0.029b |
0.016e |
0.032a |
0.022d |
0.025c |
0.01 |
|
In vitro digestibility |
||||||
|
OMD (%) |
38.28d |
45.99b |
42.60c |
44.89bc |
48.97a |
0.77 |
|
SCFA (mol) |
0.55d |
0.75a |
0.69b |
0.69b |
0.63c |
0.07 |
|
ME (MJ/kg DM) |
5.45d |
6.75a |
6.37b |
6.37b |
6.34b |
0.70 |
|
Small
case letters imply means in the same row with different superscripts are
significantly varied (p ≤ 0.05) a+b=
final gas produced, b = gas production from the insoluble fraction, c = gas
production rate constant for the insoluble fraction, UM = untreated maize
husk (control) LSM = Lentinus subnudus
degraded maize husk ,PTM = Pleurotus tuber-regium degraded maize husk, PSM = Pleurotus sajor caju
degraded maize husk, PPM= Pleurotus pulmonarius
degraded maize husk and SEM = Standard error of the mean. |
||||||
Potential
extent of gas production (a + b) ml
The potential extent
of gas production, (a + b) ml as observed in the results obtained in this
study, shows that the fungal treated maize husk were more fermented (Table 3).
This implies that the treated substrates were highly available in the rumen. It
could be seen from this result also that the entire treated maize husk had a
lower NDF compared with the untreated. Therefore, fungal treatment of maize
husk resulted in a more easily degradable substrate. This agrees with the
findings of Sommart et al.,(2000) and Nitipot and Sommart (2003) who stated that energy feeds with lower NDF
showed a higher potential extent of gas production (Table1 and Table 2). It
could also be stated that the lowest value of potential extent of gas
production (a+b) ml, obtained in the untreated maize
husk could be the result of the carbohydrate fraction having a high proportion
of lignified cell walls, with the resulting low fermentation, and thus low gas
production. This agrees with the findings of Melaku et al., (2003) who found that fibrous constituents,
especially lignin negatively influence in
vitro gas production.
In vitro organic matter digestibility (OMD) and short chain
fatty acid (SCFA)
The result of in vitro OMD is shown in Table 3. High
digestibility of organic matter was observed in all the fungal treated maize
husk, probably because the limiting lignin and crude fiber contents has been
reduced, coupled with an increase in crude protein contents. Thus, the release
of the substrate’s carbohydrate for fermentation by amylolytic
bacteria and protozoa was enhanced (Kotarski et al., 1992).This result implies that
the rumen and animal have high nutrient uptake. The result obtained for short
chain fatty acid (SCFA) (Table 3), showed significant difference (p>0.05) in
the values obtained from treated and untreated substrate. SCFA which was
generally higher in all the treated substrate implies energy availability to
the animals. A number of factors could be responsible for this, such as high
gas production in the treated substrate and this is more evident throughout the
period of fermentation. Gas production from different classes of feeds
incubated in vitro in buffered rumen
fluid is closely related to the production of SCFA which is based on
carbohydrate fermentation (Sallam et al., 2007)
Estimated metabolisable energy (ME)
Metabolisable energy was
predicted using the equation of Menke and Steingass, (1988). The estimated ME values for the
different substrates are shown in Table 3.The values obtained differed
significantly. The ME obtained for the fungal treated maize husk are similar to
those obtained for corn meal (Chumpawadee et al., 2007) and Tephrosia candida/Guinea grass mixtures (Babayemi,
2007). Menke and Steingass,
(1988) reported a strong correlation between ME values measured in vivo and predicted from 24h in vitro gas production and chemical
composition of feed. The in vitro gas
production method has been successfully used to evaluate the energy value of
several classes of feed (Getachew et al, 1998; Getachew
et al, 2002; Babayemi,
2007; Sallam et
al., 2007). Sallam et al., 2007 suggested that the in
vitro gas production system helps to better quantify nutrient utilization
and its accuracy in describing digestibility in animals has been validated in
numerous experiment.
CONCLUSIONS
The results
obtained in this study suggest that the treatment of maize husk by the
application of fungi will help in conversion of agricultural wastes to higher
quality ruminant feed thereby enhancing their digestibility by ruminants. It is
therefore recommended that more work should geared
towards this direction to harness the hidden potentials of agricultural wastes
for the benefit of the developing countries.
LITERATURED
CITED
AOAC, 1995. Official Methods of Analysis,
16th edn. (Association of Official
Analytical Chemist, Airlington, VA, USA.
Babayemi,
O. J.; D. Demeyer and V. Fievez. 2004. Nutritive
value and qualitative assessment of secondary compounds in seeds of eight
tropical browse, shrub and pulse legumes. Comm. Appl. Biol. Sci. Ghent University
69 (1): 103-110
Babayemi, O. J. 2007. In vitro fermentation characteristics
and acceptability by west African dwarf goats of some
dry season forages. Afri. J. Biotechnol. 6 (10):
1260-1265.
Belewu,
M. A. and O. C. Okhawere. 1998. Evaluation of
feeding fungi treated rice husk to ram. In Proceeding of the
25th Annual Conf. and Silver Jubilee. Nig. Soci. Anim. Prod held between 21 and 24th March,
1998 at Gateway Hotel, Abeokuta, Ogun
State.
Belewu,
M. A. and N. O Banjo. 1999. Biodelignification of
rice husk and sorghum stover by edible mushroom (Pleurotus sajor caju). Trop. J. Anim. Sci. 1 (1): 137-142.
Belewu, M. A. 2001. Conversion of
sorghum stover into feed by Trichoderma harzianum and the feeding of resulting
materials to red Sokoto goat. Bio-Science Research
Communication 13 (1): 25-30.
Belewu,
M. A.; O. Y. Afolabi, A. K. Musa and A. Z. Aderolu. 2003. Chemical composition and
biodegradability of corn cobs and Gmelina arborea saw dust colonized by edible mushroom. Proc. of
the 28th Annual Conference of the Nigerian Society for Animal
Production 28: 261-263.
Blummel,
M. and K. Becker. 1997. The degradability characteristics of fifty-four
roughages and rough neutral detergent fiber as described in vitro gas production and their relation to voluntary intake.
Brit. J. Nutr. 77: 757-768.
Broerse,
J. E. W. and B. Visser. 1996. Assessing
the Potential. In: Biotechnology:
Building on Farmers’ Knowledge. Jeske Bunders, Bertus Haverkort and Wim Hiemstra (eds).
Macmillan Education ltd. London, United Kingdom. p. 131-180.
Chen. J.; S. L. Fales, G. A.
Varga and D. J. Royse 1995. Biodegradation of
cell wall component of maize stover colonized by
white rot fungi and resulting impact on in
vitro digestibility. J. Sci. Food Agric.
68: 91-98.
Chumpawadee. S.;
A. Chantiratikul and P. Chantiratikul.
2007. Chemical composition and Nutritional evaluation of energy feeds for
ruminant using in vitro gas
production technique. Pakistan J. Nutr.
6 (6): 607-612.
Demeyee,
D. I and C. I. Van Nevel.
1975. Methanogenesis an integral part of fermentation
and it control in digestion and metabolism in ruminant. L. W. McDonald and A.
C. I. Warner eds) pp.
366-382. The University of New England Publishing Unit, Armidale, N.S.W, Australia.
Fievez,
V.; O. J. Babayemi and D. Demeyer. 2005 Estimation of
direct and indirect gas production in syringes: a tool to estimate short chain
fatty acid production requiring minimal laboratory facilities. Anim. Feed Sci.
Technol. 123 (Part 1): 197-210.
Getachew,
G.; M. Blümmel, H. P. S. Makkar
and K. 1998. Becker. In vitro gas
measuring techniques for assessment of nutritional quality of feeds: a review. 72 (3): 261-281.
Getachew. G.;
H. P. S Makkar and K. Becker. 1999. Schiometric relationship between short chain fatty acid and
in vitro gas production in presence
and absence of polyethylene glycol for tannin containing browses, EAAP
Satellite Symposium, Gas production: fermentation kinetics for feed evaluation
and to asses’ microbial activity, 18-19, August, Wageningen,
The Netherland,
Getachew. G.;
H. P. S Makkar and K. Becker. 2002. Tropical
browses: content of phenolic compounds, in
vitro gas production and stoichiometric relationship between short chain
fatty acid and in vitro gas
production. J. Agric. Sci. 139 (3): 341-352.
Jonathan, S. G. and I. O. Fasidi. 2001. Effect of
carbon, nitrogen and mineral sources on growth of Psathyyerella atroumbonata (Pegler), a Nigerian edible
mushroom. Food Chem. 72: 479-483.
Jonathan S. G.; I. O. Fasidi,
A. O. Ajayi and O. Adegeye. 2008. Biodegradation
of Nigerian wood wastes by Pleurotus tuber regium (Fries) Singer. Bioresource
Technology 99 (4): 807-811.
Kotarski,
S. F.; R. D. Waniska and K. K. Thurn. 1992. Starch
hydrolysis by the ruminal microflora.
J. Nutr. 122: 178-190.
Melaku, S,;
K. J. Peters and A. Tegegne. 2003. In vitro and in situ evaluation of selected multipurpose trees, wheat bran and Lablab purpureus
as potential feed supplements of tef (Eragrostis tef) straw.
Anim. Feed Sci. Technol. 108: 159-179.
Menke,
K. H and H. Steingass. 1988. Estimation of the
energetic feed value from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28:7-55.
Nitipot,
P and K. Sommart. 2003. Evaluation of ruminant
nutritive value of cassava starch industry by products, energy feed sources and
roughages using in vitro gas
production technique. In: Proceeding of Annual Agricultural Seminar for year,
2003,27-28 January, KKU., pp. 179-190.
Ǿrskov,
E. R and L. M. Mcdonald. 1979. The
estimation of protein degradability in the rumen from incubation measurement
weighted according to rate of passage. J. Agric. Sci. 92: 499-503.
Sallam,
S. M. A.; M. E. A Nasser, A. M. El-Waziry, I. C. S. Bueno and A. L. Abdalla. 2007. Use of an in vitro gas production technique to evaluate some ruminant
feedstuffs. J. Appl. Sci. Res. 3 (1): 34-42.
Statistical Analysis System (SAS). 1998. SAS STAT Programme, Carry, NC: SAS Institute Inc.
Sommart. K,; D. S. Parker, P. Rowlinson and
M. Wanapat. 2000. Fermentation characteristics and
microbial protein synthesis in an in
vitro system using cassava, rice straw and dried ruzi
grass as substrate. Asian-Aust. J. Anim. Sci. 13: 1084-1093.
Van Soest, P. J.; J.
B. Robertson and B. A. Lewis. 1991. Methods for dietary fiber neutral
detergent fiber and non – starch polysaccharides in relation to annual
nutrition. J. Dairy
Sci. 74: 3583-3597.
Página diseñada por Prof. Jesús Rafael
Méndez Natera
TABLA
DE CONTENIDO DE LA REVISTA CIENTÍFICA UDO AGRÍCOLA



