Revista Científica UDO Agrícola
Volumen 9. Número 3. Año 2009. Páginas: 672-680
Gonad histology in post fingerling of Tilapia guineensis
exposed to Parateq
Histología de las
gónodas en alevines de Tilapia guineensis expuestas a Parateq
Ijeoma VINCENT AKPU1 and Alex Chuks CHINDAH  2
2
1Animal and Environmental Biology, University of Port
Harcourt, Rivers State, Nigeria and
Institute of Pollution Studies,
Rivers State University of Science and Technology, Port Harcourt ,Rivers
State, Nigeria. E-mails: alexchindah@yahoo.com and alexchindah@hotmail.co.uk ![]() Corresponding author
Corresponding author
|
Received: 03/05/2009 |
First
reviewing ending: 03/27/2009 |
First
review received:
04/26/2009 |
|
Second reviewing ending: 07/20/2009 |
Second
review received: 08/15/2009 |
Accepted: 08/20/2009 |
ABSTRACT
The study investigated
the effect of Parateq on gonad morphology and changes
in gonadosomatic index of post fingerlings of Tilapia
guineensis exposed to sublethal
Parateq concentrations for 12 weeks. Initial short-
term static toxicity tests were run to determine 96 hr LC50 of Parateq in T. guineensis
which was 5.47%. The Parateq concentrations used were 0.32%, 0.63%, 1.25 % and 2.5% vol/vol parateq/ water. The histological
changes noted in the gonads of the exposed fish were inhibition of maturation
in oocytes or delay in spermatogenesis which resulted in lack of spawning. In
contrary, the four stages of spermatogenesis or oocytogenesis
were present in the control and spawning occurred. The increasing degeneration
of maturing eggs resulted in complete absence of matured egg in the female
gonads of fish exposed to the highest concentration (2.5%) of the drilling
fluid. The gonadal somatic
index values were recorded in a decreasing order toward the higher tested
concentrations. The gonadal somatic index values ranged from 2.34 to 1.25% in the
female and from 0.32 to 0.09% in male, whereas in the control, it was 2.85% for
female and 0.41% for male. The results revealed that discharge of drilling fluid such as parateq
into the environment can lead to impairment in the reproductive success of aquatic
organisms in the Niger Delta.
Key words: Gonads, Tilapia guineensis, parateq, drilling fluids, gonadal somatic index (GIS)
RESUMEN
El estudio investigó el efecto
del Parateq sobre la morfología de las gónadas y los cambios en el
índice gonadosomático de alevines
de Tilapia guineensis expuestos a concentraciones subletales de Parateq durante 12
semanas. Las pruebas iniciales de toxicidad estática a
corto plazo se reralizaron
para determinar la LC50
a las 96 hr del Parateq en T. guineensis la
cual fue 5,47%. Las
concentraciones de Parateq
usadas fueron 0,32, 0,63, 1,25 y 2,5% las cuales
correspondieron a 6,25; 12,5; 25 y 50% de la LC50 a las 96 hr, respectivamente. Los cambios histológicos observados en
las gónadas de los peces expuestos fueron: la
inhibición de la maduración de los oocitos o el retraso en la espermatogénesis,
la cual se tradujo en la
falta de desove. Por el
contrario, las cuatro
etapas de la espermatogénesis
o oocitogénesis estuvieron
presentes en el control y se produjo el desove. El incremento de la degeneración de los huevos maduros dió como resultado la ausencia total de huevos
madurados en las gónodas femeninas de los peces expuestos
a la concentración más alta
(2,5%) del fluido de perforación. Los valores
del índice gonadosomático se registraron en orden decreciente
hacia las mayores concentraciones probadas. Los valores del
índice gonadosomático oscilaron
entre 2,34 a 1,25% en las gónodas femeninas y de 0,32 a
0,09% en las masculinas, mientras en el
control, fue 2,85% para las femeninas y 0,41% para las gónadas masculinas. Los mayores
valores se obtuvieron en las gónadas femeninas comparados con las masculinas. Los
resultados revelaron que Parateq
tiene efectos deletéreos sobre los procesos reproductivos los cuales pueden
conducir a un deterioro en el éxito reproductivo de T. guineensis,
que afecte la supervivencia futura de la pesca en el delta del
Níger.
Palabras clave: Gónadas, Tilapia guineensis, parateq,
fluidos de perforación, índice gonadosomático
INTRODUCTION
The
Niger Delta is the largest wetland in Africa and among the most productive
ecosystems in the West Africa sub region and Delta is home to many important plant
and animal species that the inhabitants rely on for food and livelihood. The
wetland is characterized by oil activities (exploration and exploitation). The
attendant waste generated from the activities and occasional spills that are
discharged into the adjacent environment (OGP, 2006). One of these discharges
arising from crude oil related activities are drilling wastes (fluids) that
contain several toxic substances such as chromate, biocides, organic polymers,
hydrocarbons, heavy metals and trace elements that have the tendency to
bioaccumulation and interfere with normal biological activities of organisms
including man (Neff, 2002; PAS, 1995; Rushing et al., 1991). Drilling
fluids exposed to water may disperse or sink which will locally kill benthic organisms
by smothering them or by inhibiting physiological activities (Patin, 1999; Cranford et al., 1998; Bowmer
et al., 1996; Okpokwasili and Odokuma,
1996; Jones et al., 1991). Other aquatic resources located at the top of
the food chain have been reported to suffer severe physiological and
reproductive setback that may eventually lead to death through direct or
indirect contact via their gills, body surface and ingestion of contaminated
food (Van Dyk, 2003; Stottl
et al., 1981).
Information on drilling mud in Nigeria is limited and often not availabe to the public. However,
Soegianto et al. (2008) reported 96 hr LC50
of drilling waste between 30740 and 78271 mg
L-1 for post larvae of tiger prawn Penaeus
monodon. Similarly,
48 hr LC50
> 2000 mg L-1 for Acartia tonsa
and 72 hr EC50 >1000 mg L-1 for Corophuim volutor for Parateq was reported by Baker-Hughes (2002). In 2005, 28159 tonnes of non
aqueous drilling fluid associated with the drill cuttings were discharged into
the environment (OGP, 2006). This quantity cumulatively may have various
consequencies on the environmental integrity and biota and may be responsible for
the growing complaint of low fish yield by the fisher folks. Recently, the steady declining yield of fin and non
fin fishes has generated lots of concern attributed to oil industrial
activities including drilling waste discharges (Kinigoma,
2001; Wills 2000; Patin, 1999). In the Niger delta
region, efforts to the effect of oil activities on environment and biota had been tailored mostly on the effect of
crude oil spills on water quality (IPS, 1989, 1990; RPI, 1985), phytoplankton (Chindah and Braide, 2001; NDES,
2000), periphyton (Chindah,
1998; Pudo and Fubara,
1988), benthos (Ekweozor et al., 1987; Ekweozor and Snowden 1985), gastropod (Chindah
et al., 2000; Dambo, 1992),
crustacean (Chindah et a.l,
2004), vegetation (Osuji and Ezebuiro,
2006: Obot et
al., 1992) and fish (NDES, 2000; IPS, 1989
& 1990; Powell, 1987).
Despite the huge drilling activities and the attendant drilling waste
generated and discharged, little is known on the sublethal effect of drilling fluid such as Parateq that is commonly used in wetlands areas of the Niger
Delta region. It is on the basis of this gap in knowledge that this study was
undertaken in order to evaluate the possible effect of the drilling fluid on
the development (reproductive) of the most common and widely distributed fish
species in the region.
MATERIALS
AND METHODS
The Parateq is a synthetic
based fluid obtained from Baker Hughes Nigeria Limited made up of mosaic of complex chemical compounds and including heavy metals (Table 1). It is commonly
used for drilling operations worldwide. Post fingerlings (7.21 - 7.25cm
/ >10.5g) of T. guineensis used were
collected from the African Regional Aquaculture centre
(ARAC), Buguma, Rivers State Nigeria.
|
Table 1.
Physical and chemical characteristics of Parateq |
||
|
Parameter |
Concentration |
|
|
Water |
26.20 |
% |
|
Base Fluid |
73.80 |
% |
|
Organophilic lignite(carbongel 11) |
12.00 |
ppd |
|
Organophilic clay (omniplex)
|
2.16 |
ppd |
|
Lime |
3.00 |
ppd |
|
CaCl2 |
32.78 |
ppd |
|
Barite |
105.26 |
ppd |
|
Polyaminated fatty acid (omnimul) |
8.62 |
ppd |
|
pH |
6.76 |
|
|
Total Solid |
587 |
mg/g |
|
Total organic carbon |
1.65 |
mg/ g |
|
Chloride |
0.63 |
mg/ g |
|
Nitrate |
1.60 |
mg/g |
|
Total hydrocarbon |
41.00 |
mg/g |
|
Lead
|
2.16 |
ppm |
|
Manganese |
2.05 |
ppm |
|
Zinc
|
5.82 |
ppm |
|
Cadmium |
0.00 |
ppm |
|
Chromium |
0.09 |
ppm |
|
Barium |
0.004 |
ppm |
The fish were transported in the early hours of the
day to the laboratory in air bags with the pond water from the fish farm to
avoid heat exertion.
In the laboratory, the fish were transferred
immediately to the holding tanks [120 x 120 x 120cm]. The holding tanks were
aerated, cleaned and the water renewed regularly (Reish
and Oshida, 1986). Fish were fed twice daily with
NIOMR feed (35% protein). During the acclimatization period, the fish were
gradually subjected to the dilution water until they could survive in the
uncontaminated dilution water without showing signs of stress, such as discolouration or unusual behaviour.
At the end of the acclimatization period, all the fish that were disease free,
without any signs of stress, or damage were used for the experiment. Mortality
during the holding period was less than one percent of the whole population.
The bioassay was conducted in ten
Initial 96 hr short lethality test
was carried out for the post fingerlings exposed to 0%, 2%, 4%, 8% and 10% of Parateq in water to
determine the median lethal concentration (LC50)
(concentration of drilling fluid in water that will
kill 50% of the fish population in 96 hours) as
in Ca1/EPA,( 2004). The LC50 was
calculated based on the probit analysis through which five sublethal
concentrations 2.5 %, 1.25 %, 0.63 %, 0.32 % and control were obtained in a
volume to volume ratio (Reish and Oshida,
1986; Vincent-Akpu, 2001). Note,
1% = 1000ml/L.
A group of ten fish was randomly exposed to the
different concentrations of the drilling fluids. Healthy fish were assigned to
the aquaria and screened with a mosquito net to prevent fish escape. Exposure lasted for 12 weeks, during which
freshly prepared test solutions were made weekly as the water is changed and
tanks cleaned (Reisha and Oshida,
1986). The fish were fed with NIOMR feed at 4% of their weight twice daily.
Water parameters (Temperature, pH, DO and alkalinity) of the test solution were
monitored weekly throughout the duration of the experiment (APHA, 1998). At the
end of the 12 weeks, four fish were sacrificed
by a sharp blow to the head, weighted and the total length recorded. The gonads
were excised from the fish and weighed. The gonodal
somatic index (GIS) was calculated from gonad weight
x 100 / body weight.
Care was taken not to squeeze any of the tissues and
processed by methods given by Golder (1997) and Wester et al. (2003).
The tissues were placed in a tissue cassette and
fixed immediately in 10% neutral formalin in nalgene
container for 36hr to avoid post–mortem changes.
The samples were washed in running tap and
dehydrated in a graded series of industrial Methylated spirit (30, 50, 70, 80,
90 and 100 %) for specific time periods. The samples were then transferred to xylene
for 5 minutes, until transparent (clear) and later transferred to 60oC
oven.
The samples were infiltrated and imbedded in
paraffin wax blocks. After cooling, the imbedded samples were sectioned (5µm
thick) using a wax microtome. The sample sections were stretched with an
albumin and distilled water solution, mounted on glass microscopic slides and
air dried. The dried sections were stained with Haemotoxylin
and Eosin (H&E) staining techniques. Stained sections were then mounted
with cover slide using entellan. Each slide was
reviewed microscopically without any knowledge of its individual treatment and
a histological report prepared. Photomicrographs were taken to illustrate some
of the tissue pathology recorded.
The median lethal
concentration (LC50) and median
lethal time(LT50) were
calculated using probit analysis. The significant
differences among the treatments were assessed using analysis of variance (ANOVA) and were considered to
be significant if pP<0.05 while T-test was used to determine
mean difference between the physicochemical
parameters.
RESULTS
The
level of physicochemical parameters determined during the experiment at the
various concentrations did not vary significantly (p < 0.05, n = 4) from those of the
control (27.3 ± 0.04 °C; 4.26 ± 0.42 mg L-1; 22.31 ± 0.48 mg L-1; 7.01 ± 0.09 for temperature,
dissolved oxygen, alkalinity and pH respectively).
In the short term
lethality test, mortality increased with increased in concentration. Mortality in % was
transformed to probit (5 corresponds to 50% mortality). While the time and
concentrations were transformed into logarithrim(log). This gave 24, 48, 72 and 96 hr LC50 of 11.56%, 9.24%, 7.24% and
5.47% respectively for Parateq (Figure 1). The LT50 were
102.15 hr for 2%, 93.63 hr for 4%, 65.84 hr for 8% and 55.51 hr for 10% (Figure 2).
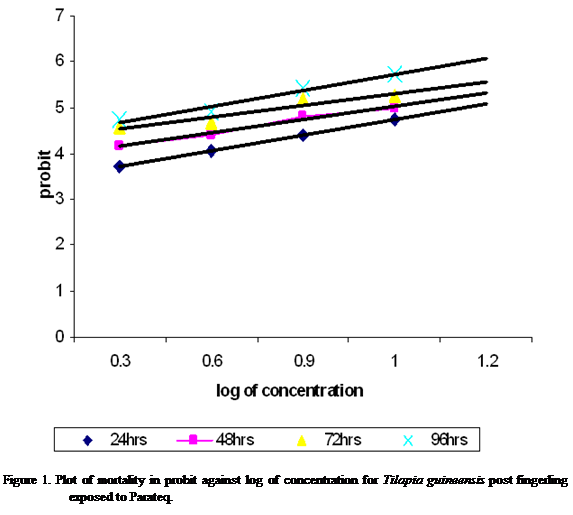
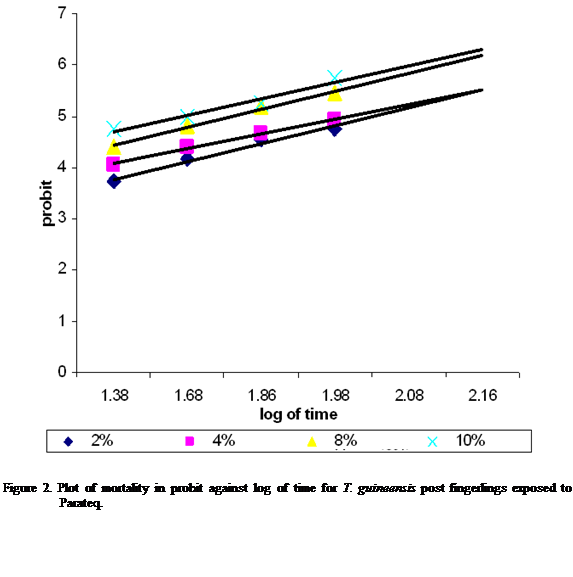
The
mean gonadal
somatic
index (GIS) of post fingerlings exposed to different concentrations of Parateq is presented
in Table 2. The GSI
values decreases as the concentration of Parateq
increases with exception of the control which had 2.85 and 0.41% for female and
male gonads respectively. The analysis of variance showed that there was a
significant difference at p<
|
Table
2. Effects of Parateq
on gonadal somatic index of the post
fingerling of Tilapia guineensis |
||
|
Concentrations (%) |
Female |
Male |
|
0 |
2.85 |
0.41 |
|
0.31 |
2.34 |
0.32 |
|
0.63 |
1.97 |
0.21 |
|
1.25 |
1.75 * |
0.15 |
|
2.50 |
1.25 * |
0.09 * |
|
*
significant difference with the controls (P< 0.05, n = 8) |
||
Gross examination
of the gonads gave no indication of swelling or discolouration.
There was no discernible difference in the size of the left and right lobes of
the gonads.
Four developmental
stages were distinguished as primary oogonia,
secondary oogonia, primary oocyte and secondary
oocyte with the characteristically prominent zona pellucida in female gonads while spermatogonia,
spermatocyte, spermatides and spermatozoan
were found in the male gonads. Successful spawning occurred in the control weeks before the
sampling (Plate 1). The female gonads in the control consist of lamellae filled with ova in various
stages of development and the testis contains cluster of numerous spermatogenic cells (cysts) at various developmental stages
in mature seminiferous tubule. The fibrous seminiferous tubule are intact and
the tubules numerous. However, most of the cells in the seminiferous tubule
were at the last stage of development mainly spermatocytes and few spermatids.
The
histological changes observed in the exposed female gonads were inhibition of maturation in
oocytes coupled with increasing number of atretic follicle. Parateq affected the gonads in a
dose dependent manner. The severity of pathological changes became more
intense as the concentration increases while there was complete absence of
matured eggs in the female gonads of fish exposed to the highest concentration
of Parateq. In ovaries of treated fish exposed to 0.32% Parateq, no discernible difference between treatment and control
could be seen. More frequency of immature egg cells was observed in 0.63% while gonad in 1.25% Parateq contained many immature follicles accompanied by a slight
decrease of early vitellogenic and increase in relative number of oogonia. At maximum
concentration (2.5%) of Parateq, gonad development
was inhibited which was reflected in decreased oocyte growth and high incidence
of atresic follicle resulting in complete fusion of
two follicles (Plate 1A). No spawning occurred in all fish exposed to Parateq.
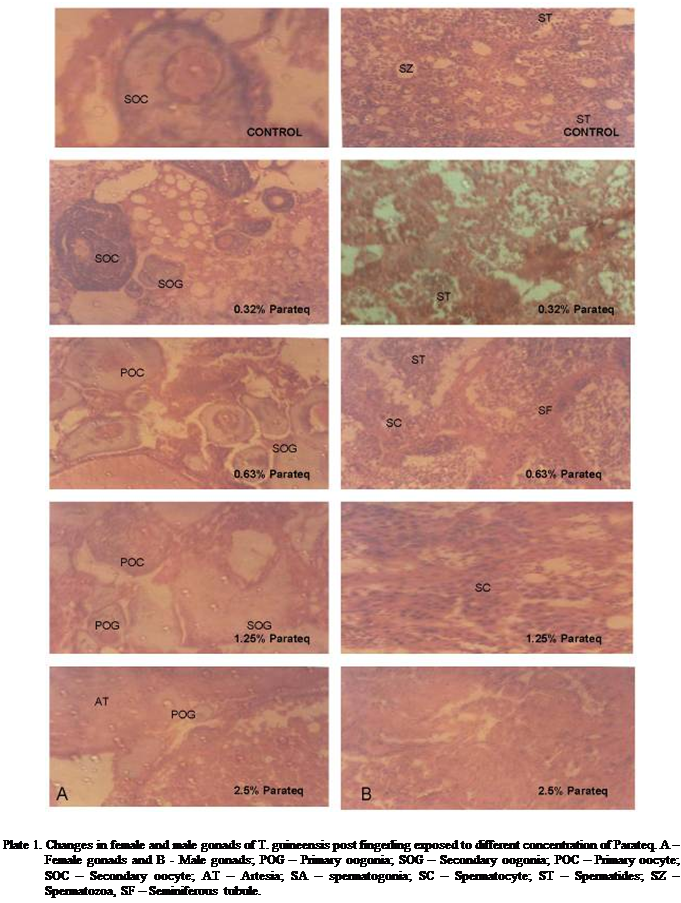
Parateq was observed to
have induced
a dose-dependent inhibition of spermatogenesis in testes of male fish. The
gonads of the exposed fish at the higher
concentrations were clearly distinguishable from the control gonads. The second
lowest exposure concentration testes possessed reduced tubules with fewer
spermatocytic stages. This effect increased with increasing concentration and
at 0.63% and 1.25%, spermatocytic stages decreased and spermatogenesis appeared
to be inhibited. The testes were characterised by enlarged semiferous tubule
filled with spermatid and enlarged spermatocytes with a much greater number of
mature sperm contained with in and the relative lack of germinal epithelium and
primary and secondary spermtocyte. Post fingerlings of T. guineensis
exposed to varying concentrations of Parateq had
multiple lesions which is characterised by
degeneration of germ cells and depletion of the numbers of seminiferous
tubules. There was tendency to decrease in frequency of progressed stages (spermatozoan and spermatides) and
increased presence of early developmental stages was observed as the
concentration increases (Plate 1B).
In the fish exposed to the highest concentration of Parateq, inhibition of spermatogenesis was observed which
was characterised by absent of matured sperm and
atrophy of the seminiferous tubule.
DISCUSSION
The
values used for sublethal testing are very much
dependent on the acute toxicity tests performed, so that extrapolation using LC50 values needs to be done with
caution. The percentage mortality, which increased progressively with increase
in concentration of drilling fluid over time of exposure, is in agreement with
previous findings (OGP, 2003; Bowmer et al., 1996). However, the acute
toxicity was relatively low. This is probably because the test was not renewed
daily during the 96hr bioassay period. Neff et al. (1981) noted that
if aqueous mud fraction was renewed daily, its toxicity will increase
several-fold, demonstrating that the toxic components may be lost from solution
by volatilization.
The
48LC50 values for Parateq (9.24% = 92.4ml/L) obtained in this study was
higher compared to what was obtained in Baker Hughes (2002) for the same
drilling fluid which was 48hr LC50 > 2000 mg L-1 for A. tonsa
and 72 hr EC50
>1000 mg L-1 for C. volutor.
Using a
conventional toxicity rating classification system as a method for ranking and
comparing relative toxicities of drilling fluids (Swan et al., 1994). Parateq with 96 hr LC50 of 0.54% or 5400
ml/L can be said to be slightly toxic
since it lies within 1000 - 10000 mg L-1.
Aquatic pollution is therefore less related to acute
toxicity than to sublethal and long-term effects
which are difficult to detect. Early toxic effects of pollution may however be
on cellular or tissue level before significant changes can be identified in behaviour or external appearance (Martinez, et al., 2004; Terio, 2004; Van Dyk, 2003).
Sublethal exposure to the drilling fluid resulted in noticeable
effect on reproduction of T. guineensis. Four
main developmental stages of oogenesis and four stages of spermatogenesis as
modified by Stottl et al (1981) were
identified. The developmental stages were characterized by the abundance of the
stages of oogenesis which are primary oogonia,
secondary oogonia, primary oocyte and secondary
oocyte and spermatogonia, spermatocyte, spermatides and spermatozoan were
found in spermatogenesis.
The
reproductive success of the gonads exposed to Parateq
was affected as shown by a decrease in relative weight (GSI) and a decrease
frequency of mature oocytes or spermatocyte. This is similar to delay maturation and inpaired reproductive
success observed by Bowmer et al (1996) when Cardium edule was
exposed to drill cuttings using a long term model ecosystem bioassay.
Similarly, Bhuiyan et al (2001) observed ovarian
damage such as complete blockage and dissolution of ovigerous
lamella in Channa punctatus
exposed to sumithion.
The
concentration dependent decrease in frequency of matured oocyte or spermatocyte
in the different concentrations was due to inhibition of spermatogenesis or
oogenesis. Disturbed oocyte development, or at least a delay in the final
maturation, was revealed by the large proportion of unovulated
yolk egg especially in the ovaries of the most severe exposure.
Vuorinen et al (2003) attributed this delay to
stress-induced increase in cortisol concentration which in turn suppresses
gonadotrophic hormone-(GTH) - Stimulated testosterone and 17β-estradiol production in peak vitellogenic
follicles in Coregonus albula
L. This was supported by Wester et al
(2003) in the study of the effects of hormone in Zebra fish. In spite of the
fact that the effect of parateq on hormone was not
investigated in this study, however the propensity observed in male T. guineensis, with delay in spermatogenesis was seen as
implying that there is tendency toward a lower gonadal somatic index (GSI)
in the exposed group with the observed retardation in spermatogenesis. Reduced
androgen production might be behind the retarded spermatogenesis (Wester et al, 2003). Spermatogonia apparently did not under a further
differentiation to spermatogenic cysts and spermatids. This possibly indicates
a cessation of milt production revealed
by the accumulation of large spermatocyte in the lumen of enlarged semiferous
tubules and lack of intermediate stages. However, the possibility has to be
concidered that the stand still in milt production is not pathological but
rather the response of males to the stop of spawning activities in females. It is interesting to note that
the histological response of testes to parateq is much like that observed in
previous studies with 3-benzylidene camphor by Kunz et al (2006). A 21 days exposre to 3-benzylidene inhibited testical
development, but showed less degeneration on fat head minnow (Pimephales promelas).
Spawning
occurred in the control 3 weeks before sampling, which can explain the lack of
many spermatozoa and secondary oocyte. Cranford et al (1998) observed
that fertilization success of the sperm and egg of haddock, sea scallop and
lobster were not significantly affected when exposed to water based drilling
fluids concentration below 100 mg L-1. In contrast,
spawning did not occur in the post fingerlings exposed to various
concentrations of Parateq even in the lowest
concentration of 0.32%, indicating that reproductive process was hindered at
one stage or the other.
The
effect of the drilling fluid on GSI of T. guineensis
was influenced by the level of gonadal activities as indicated by comparison of
fish with or without the treatment. The decrease of GSI values observed in the
drilling fluid-treated fish was associated with the concentration of the
drilling fluid and pathological changes as shown by a decrease in the stages of
gonadal maturation and increased frequency of histological changes.
The results of the present study
revealed that Parateq has deleterious effects on the
reproductive processes which could lead to impairment in the reproductive success of T.
guineensis. Reproductive process in fishes
involve changes in weight and structure of gonads, using the gonadal somatic index
(expression of gonad weight as a percentage of the body weight) and
histological changes in the gonads, can provide insight to any abnormality in
the fish health. The inhibition of spermatogenesis or oogenesis coupled with high incidence of atresic follicle and lack of spawning observed in this study indicates
its usefulness as indicator of physiological disturbances (Wester, et al 2003). Histological
response of the fish gonads to environmental stress has shown to be a biomarker
indicative tool to assist in the bio-monitoring process of aquatic ecosystems (Byuiyan et al, 2001). Therefore, with the
extensive exploration drilling and production that occur in the Niger Delta,
their ecological impacts must always be kept in mind.
ACKNOWLEDGEMENTS
We thank sincerely thank the
staff of the Institute of Pollution Studies Rivers State University of Science
and Technology and Ikoro Udona and Awaini Osuamkpe in particular for providing
access to laboratory facilities. We express profound gratitude to Solomon
Braide, whose critical review of an earlier version of the manuscript helped to
make this work a reality. More thanks are also due to the unanimous reviewers
for the helpful comments and suggestions on the manuscript
LITERATURE CITED
American Public Health Association (APHA). 1998. Standard methods for the examination of water
and wastewater, 20th ed. APHA-AWWA-WPCF. Washington D.C. 1220p.
Baker-Hughes.
2002. Baker Hughes INTEQ. Parateq safety data
sheet. 4p.
Bhuiyan, A. S.; B. Nesa and Q. Nessa. 2001.
Effects of Sumithion on the histological changes of
spotted murrel, Channa
punctatus (Bloch) Pak. J. Biol. Sci. 4 (10): 1288-1290.
Bowmer, C. T.; S. Gimeno, E. M. Foekema and N. H. B.
M. Kaag. 1996. An
environmental evaluation of cleaned drill cuttings using a long-term
model ecosystem bioassay. In: The
physical and biological effects of processed oily drill cuttings (summary
report) E & P Forum Joint Study. p. 64-78.
Cal/EPA, 2004. Overview of fresh water and marine
toxicity tests: A technical tool for ecological risk
assessment. Cal/EPA Office of Environmental
Health Hazard Assessment and Dept. of Environmental Toxicology, University of
California. Davis. USA. 147p.
Canford, P.; K. Querback, J. Maillet, P. Grants,
C. Taggart and K. Ice. 1998. Effect of
water based drilling mud on early life stages of haddock, lobster and sea
scallop. Canada – Nova Scotia offshore petroleum board archives. p. 1-2.
Chindah , A.
C. 1998. The effect of industrial activities on the periphyton
community of upper New Calabar River, Nigeria. Water
Res. 32 (4): 1137 -1143.
Chindah, A. C.; S. A. Braide and E. Nduaguibe. 2000. Tolerance
of periwinkle (Tympanotonus fuscatus and shrimp (Palemonetes africanus
Balsa) to waste water from bonny light crude oil tank farm. Polish J.
Environmental Protection and Natural Resources. 21/22: 61-72.
Chindah, A. C.
and S. A. Braide. 2001. Crude oil spill on the
phytoplankton community of a swamp forest stream 19 years after spill. Afr. J. of
Environ. Stud. 2: 1-8.
Chindah, A. C.; O. C. Sibeudu,
S. A. Braide and C. Onyebuchi. 2004. Distribution of hydrocarbons and
heavy metals in sediment and a crustacean (Penaeus notialis) from the Bonny/New
Calabar River Estuary. Niger
Delta. Afri. J. of Environmental Assessment and
Management. 9: 1-17.
Dambo, W. B.
1992. Tolerance of the periwinkles Pachymelina
aurita (Muller) and Tympanotonus
fuscatus (Linne) to
refined oils. J.
Environmental Pollution. 79: 293-296
Ekweozor, I. K.
E. and R. J. Snowden 1985. The studies of the impact of a minor oil spilage in the upper Bonny estuary. Paper presented at
NNPC/FHME. International Seminar on the Petroleum Industry and the Nigerian
Environment. 12-13th Nov. 1985, Kaduna, Nigeria. p.112-119
Ekweozor, I. K.
E.; M. Ugbomeh
and E. I Ombu.
1987. The effect of chronic oil pollution in the central bonny estuary. Proc. of 1987
seminar on the Petroluem on the Petroleum industry
and the Nigerian Environment. Nov 9- 12, Port Harcourt. p. 198-207.
Golder, 1997.
Golder Technical Procedures. Fish Health
Assessment-Methods. Golder
Technical paper. 28p.
IPS 1989. Environmental data acquisition at some NNPC
operational areas. III Biological studies: Institute of Pollution Studies,
Rivers State University of Science and Technology Port Harcourt, Nigeria. RSUST/IPS/TR/89/03. 393p.
IPS 1990. Ecological post impact study
of Ebubu-Ochani oil spillage. Institute of Pollution
Studies, Rivers State University of Science and Technology Port Harcourt , Nigeria . RSUST/IPS/TR/90/02. 23p.
Jones, F. V; J. H. Rushing and M.
A. Churan 1991. The chronic
toxicity of mineral oil-wet and synthetic liquid-wet cuttings on an estuarine
fish, Fundulus grandis.
In: First international conference on Health, Safety and Environment. The
Hague, The Netherlands, November 10-14, SPE 23497. p. 721-730.
Kinigoma, B. S.
2001. Effect of drilling fluid additives on the Niger Delta Environment:- A
case study of the Soku oil fields. J. Appl. Sci.
Environment Management. 5 (1): 57-61.
Kunz, P. Y.; T. Gries and K. Fent 2006. The ultraviolet filter
3-Benzylidene Camphor adversely affects repeoduction in fat head minnow (Pimephales promelas). Toxicological Sci. 93 (2): 311- 321.
Martinez, C. B. R.; M. Y. Nagae, C. T. Zaia and D. A. M. Zaia 2004. Acute
morphological and physiological effects of lead in the neotropical
fish Prochilodus lineatus.
Braz. J. Biol
64 (4): 797-807.
Niger Delta Environmental Survey (NDES). 2000.
Ecological Zonation and Habitat
classification Phase 2 Report, Vol.1, i-xxxiii, p. 1- 66.
Neff, J. M. 2002. Fate and effects of mercury from oil
and gas exploration and production operations in the marine environment prepared for API. Washington D. C. 135p.
Neff, J. M.; R. S. Carr and W. L. Mc Culloch 1981.
Acute toxicity of a used chrome lignosulphonate
drilling mud to several species of marine invertebrate. Marine Environ Res. 4:
251-266.
Oil and Gas Producer (OGP). 2003. Environmental
aspects of the use and disposal of non-aqueous drilling fluids associated with
offshore oil and gas operations. Report No. 342. 103p.
Oil and Gas Producer (OGP) 2006.
Environmental performance in the E & P industry – 2005 data. Report
No. 383. 46p.
Okpokwasili, G. C.
and L. O. Odokuma 1996. Response of Nitrobacter to toxicity of drilling chemicals. J. Pet. Sci.
Eng 16: 81-87.
Osuji, L. C.
and P. E. Ezebuiro 2006.
Hydrocarbon contamination of a typical mangrove floor in Niger Delta, Nigeria.
Int. J. Environs. Sci. Tech. 3 (3): 313-320.
Obot, E. A.; A. C. Chindah and S. A. Braide 1992. Vegetation recovery and herbaceous production
in a fresh water wetland 19 years after a major oil spill. Afr. J. Ecol. 30: 149-156.
Pollution Assessment Study (PAS) 1995.
The Niger Delta, Nigeria. Prepared for the World Bank. Carl Bro. International.
91p.
Patin, S. A.
1999. Environmental Impact of the offshore Oil and Gas industry. Ecomonitor Publishing, East Northport, New York, USA.
Powell C. B. 1987. Effects of
freshwater oilspill on fish and fisheries.
Proceedings of 1987 seminar on Petrolem Industry and
the Nigerian environment.
Pudo, J. and Fubara, D. M. 1988. Studies on the periphyton
algae in the petroleum oil spillage area of the Niger Delta aquatic system. Verh. Inter. Ver. Limnol. 23:
2259-2261.
Reish, D. J.
and P. S. Oshida. 1986. Manual of methods in aquatic
environmental research. Part 10. Short-term static bioassays. FAO fish. Tech
Pap (247) 62p.
RPI 1985. Environmental baseline studies for the
establishment of control criteria and standards against petroleum related
pollution in Nigeria. Research Planning Institute, South Carolina USA, i-xiii,
45p.
Rushing, J. H.; M. A. Churan and F. V. Jones 1991).
Bioaccumulation from mineral oil-wet and synthetic liquid-wet cuttings in an
estuarine fish, Fundulus grandis. In:
first International Conference on Health, Safety and Environment, The Hague,
The Netherlands. SPE 23350. p. 10-14.
Soegianto, A.; I. Bambang and A. Mochammad. 2008.Toxicity
of drilling waste and its impact on gill structure of post larvae of tiger
prawn (Penaeus monodon). Global Journal of
Environmental Resource 2 (1): 36 - 41.
Stottl, G. G.; N. H. Mc Arthur, R. Tarpley, V. Jacobs
and V. Sis R. F. 1981. Histopathology of ovaries of fish from petroleum
production and control sites in the Gulf of Mexico. J. Fish Biol. 18: 264-269.
Swan, J. M.; J. M.
Neff and P. C. Young. 1994. Environmental
implications of offshore oil and gas development in Australia - the findings of an independent scientific review, Australian
Petroleum Exploration Association, Sydney, 157 p.
Terio, K. A. 2004.
Comparative Inflammatory Response of Non-mammalian Vertebrates: Robbins and Cotran for the birds and fish respectively. IVTS. Ithaca.
New York. p. 1104 -1225.
Van Dyk, J. C. 2003.
Histological changes in the liver of Oreochromis
mossambic (cichlidae)
after exposure to cadmium and zinc. M. S. thesis. Aquatic Health. Rank
Afrikaans University. South Africa. 150p.
Vincent-Akpu, I. F. 2001.
Toxicity of two pesticides, cypermethrin and chlorophyfos on juvenile of Tilapia guineensis.
M. S. thesis. University of Port Harcourt, Nigeria. 115p.
Vuorinen, P. J.; M. Keinanen, S. Peuranen and C. Tigerstedt. 2003. Reproduction, blood and
plasma parameters and gill histology of Vendace (Coregonus albula)
in Long – Term Exposure to Acidity and Aluminium. Ecotox. and Environ.
Safety 54: 255-276.
Wester, P. W.; E. J van den Brandhof, J. H. Vos and L. T.
M van der Ven. 2003. Identification of endocrine disrupter
effects in the aquatic environment a partial life cycle assay in zebra fish.
RIVM report 64092001/2003. 112p.
Wills, J. 2000. Muddied waters. A Survey of Oil Field
Drilling Wastes and Disposal Techniques to Reduce the Ecological Impact of Sea
Dumping. Sakhalin Environmental Watch. 139p.
Página diseñada por Prof. Jesús Rafael Méndez Natera
TABLA DE CONTENIDO DE LA REVISTA CIENTÍFICA UDO
AGRÍCOLA



