Revista Científica UDO Agrícola Volumen 8.
Número 1. Año 2008. Páginas: 143-153
Response of Sarotherodon melanotheron
Rüppell (1852) in the Niger Delta wetland, Nigeria to changes in pH
Respuesta del pez óseo común Sarotherodon melanotheron Rüppell (1852) en los humedales del Delta del Níger,
Nigeria a los cambios de pH
Alex Chuks CHINDAH ![]() 1, Amabaraye Solomon BRAIDE1 and Olisa ORANYE2
1, Amabaraye Solomon BRAIDE1 and Olisa ORANYE2
1Institute
of Pollution Studies. Rivers State University of Science and Technology. P M B
5080, Port Harcourt, Rivers State,
Nigeria and 2Department of Petroleum Resources. Nigerian
National Petroleum Corporation, Moscow Road, Port Harcourt. E-mails: E-mails:
alexchindah@yahoo.com and alexchindah@hotmail.co.uk ![]() Corresponding
author
Corresponding
author
|
Received: 04/24/2008 |
First reviewing ending: 06/20/2008 |
|
First review received: 10/16/2008 |
Accepted: 10/27/2008 |
ABSTRACT
The response of a common Niger Delta wetland Cichlid (Sarotherodon melanotheron Rüppell) to changes in pH was assessed under renewal
static assssy in the laboratory using physical attributes such as swimming and
body movement (including opercular and fin movement), mucus deposition at the
inner opercular cover in addition to hematological parameters such as
erythrocyte and leucocyte numbers and hemotocrit values of the fish. Fishes
were exposed to varying adjusted pH regimes of 3.6, 4.0, 5.0, 6.0, 7.0
and 8.0 by acidification and liming employing recommended standard procedures.
The result demonstrated that the fishes surfaced to the top of the water column
regular in erratic and unsteady manner with increased acid of the water. Fish’s
responses to different pH through hematological parameters as blood glucose, red blood cells and hematocrit)
are also discussed.
Key
words: Sarotherodon melanotheron, pH changes, hematological parameters, hematocrit
RESUMEN
La respuesta del pez Sarotherodon melanotheron (Rüppell)
a cambios en el pH se determinaron bajo condición semi estática en el
laboratorio usando atributos físicos tales como movimientos de natación y del
cuerpo (incluyendo movimiento opercular y de aletas), deposición de moco en la
cubierta opercular interna en adición a los caracteres hematológicos tales como
número de eritrocitos y leucocitos y valores de hematocritos del pez. S. melanotheron se expuso a los
regímenes de pH de 3,6; 4,0; 5,0; 6,0; 7,0 y 8,0 empleando procedimientos
estándares recomendados. El resultó demostró que los peces estuvieron en el
tope de la columna de agua de una manera errática e inestable con el incremento
de la acidez. La respuesta de los peces a las variaciones del pH a través del
fluido corporal (caracteres hematológicos –glucosa en la sangre, glóbulos rojos
y hematocritos) es también discutida.
Palabras
claves: Sarotherodon melanothero, cambios de pH, caracteres hematológicos, hematocritos
INTRODUCTION
Anthropologic activities change the
environment quality. Magnitude of the resultant effects varies depending on the
type, extent and quality of impacting conditions. The alterations have
threatened functional attributes and the existence of aquatic organisms
especially fish (FAO, 1997, Chindah and Hart 2000).
Activities such as construction, clearing
of vegetation, dumping of solid wastes, industrial and municipal effluents
especially in the wetlands acidify of the water body. Other common industrial
activities in the Niger Delta region such as gas flaring amongst others yield
combustion products such as CO2, NO2, CO, water vapour
and soot or carbon particles, heavy metals and incombustibles in the atmosphere
that are ionized and become chemically reactive as free radicals (Ibiebele,
1987). These chemicals and particles in presence of rainwater and water vapour,
readily form acids (and other corrosive chemical compounds), which build up in
the atmosphere and are eventually washed out as acid rain, altering the pH of
the recipient medium. The presence of
several industrial plants such as refineries, flow stations, Petrochemical,
Liquefied Natural Gas and Fertilizer Plants in the region with their respective
flare stacks deposit large volumes of gas into the atmosphere. In addition, the
effluent arising from these industrial activities is discharged into
surrounding water bodies thus contributing significantly to the alteration of
the pH of the aqueous medium (Spiff and Horsefall, 1998).
Changes in the pH and redox-potential of
the aquatic environment are of great concern to all stake holders such as the
Industries (IDS), Community Based Organizations (CBO), Academia (AC),
Governmental Agencies (GA) and Non Governmental Agencies (NGO) following the
declining catch of fin and non-fin fish species which had often times been
attributed to altered water quality especially changes in pH (Spiff and
Horsefall 1998). Some studies have implicated nutrient enrichment, increased
heavy metals, and presence of pesticides to the reduced pH of the aquatic
medium (FAO 1997; Brown et al, 1984;
Sadler and Lynam, 1987). Physical (movement of body, fins, opercular bones) and
physiological (hematological parameters) attributes of fishes have been used as
indicator of fish responses to its externalities (Casillas and Smith, 1977). It is consequently crucial to use these
attributes of fish in the monitoring of fishes responses to increasingly acidic
pH levels.
Despite the threat posed by changes in pH
in the aquatic systems of the Niger Delta region, little has been reported on
its effect on fishes (Spiff and Horsefall 1998).
In an attempt to bridge the existing gap
on the effects of reduced pH on fish, physical and hematological parameters
were considered. In order to achieve this,
Sarotherodon melanotheron a freshwater species was exposed to low pH
regimes, to determine changes in hematological parameters (erythrocyte,
leucocyte, and hematocrit values).
MATERIALS AND METHODS
Description of test species
The tested fish species
is a fresh water type of the family Cichlidae ‑ Sarotherodon melanotheron (Ruppel,
1852) that is commonly found in waters of the Niger Delta contributing in a
high percentage to the artisanal fisheries of Southern Nigeria as their oily
flesh tissue is greatly relished by most local people (Akiri, 1987 and Pudo et al.,1990).
This species is characterized by deep pre-orbital bones, paternal mouth
brooding habit and preference for brackish water environment as against the
species such as Tilapia zilli
(Trewavas, 1983). Colouration varies with location, sexual activity and changes
with environmental background indicting
a form of mimicry of the immediate habitat. The black spots on the chin
and throat vary considerably both within and among populations. Mature males
often have a proportionately large head caused by mouth brood (Akiri, 1987
and Pudo et al.,1990).
Sample collection
Sarotherodon
melanotheron of almost uniform length
(5.7 ± 0.5cm) and weight 3.6± 0.4g) were collected with drag-nets from
freshwater fishpond at African Regional Aquacultural Centre Aluu, Portharcourt.
Samples were sorted to different size classes using standard length (cm) and weight (using a OHAUS Triple
Beam Balance - g) and sex of the fish not accounted for during the experiment.
In the field, fishes considered healthy on the basis of their appearance and
absence of obvious signs of stress were transferred to large holding tanks for
immediate transportation to the laboratory (Kori-Siakpere, 1985).
Experimentation
Acclimatization of test species laboratory conditions
In the laboratory, 750
individuals collected were transferred and equally distributed using portable
hand net into twenty five (25) 80‑litre capacity glass tanks (i.e. 30
fishes in each) with each tank measuring 65 cm x 35 cm x 35 cm and filled with
50 L of water from the natural environment. Portable aerating pumps were
connected to each tank for oxygenation. A 1.3 KVA Honda generating set was on
standby as alternative power supply source, of. A 1.91 cm nylon mesh was
carefully positioned at the top of each tank to prevent fish escape as a result
of jumping. Fishes were observed daily and any dead, injured or morbid ones
were removed immediately. They were fed twice daily between 0900 hrs and 1000
hrs, and between 1500hrs and 1600hrs on a special diet of 30% crude protein
marshed fish feed and kept in this condition for 2 weeks (Kori-Siakpere, 1985). These fish formed the ready stock for the 96
hr LC50 and the treatment schedule.
96 hr LC50 test
A 96 hr LC50 test was carried out for the
selected fish species within an acute toxicity range of pH 2.5 to 4.0. The test
was to serve as a guide in determining the lower‑limit pH value for the
study (Chindah et. al 2004).
Twenty of each already
acclimatized samples were introduced into each of the 15 tanks containing 50
litres of fresh water. Tanks were maintained at five pH values ‑ 2.5,
3.0, 3.3, 3.6, and 4.0 by adding concentrated H2SO4 (BDH,
GR grade). The tank for each pH value was setup in triplicate. The acid
dropping system (Dheer et al., 1987)
was done for all tanks to ensure constant pH during the 96‑hour exposure
period.
Concentrated H2SO4 (96% Stock) was dropped from a 2ml pipette into a beaker
containing one litre of natural water and then using a CORNING pH meter model
7, the desired pH for the volume of water was attained. The volume of H2SO4 required to adjusting 50 litres of water was applied and
thoroughly stirred for few seconds and re-measured with a pH meter to ensure
the desired pH value.
The tanks were
maintained for 96 hours. They were cleaned and water changed after 2‑day
interval when the concentration of H2SO4 (or alkali) was
adjusted to counteract the pH drift due to release of excretory products and
other metabolites. Continuous aeration
was maintained throughout the experimental period to avoid, the building up of
any free CO2 which is toxic and capable of altering the pH in the
tank. Observations were made every 24 hours and numbers of dead and live fishes
were recorded. Fishes were considered dead when they lost their equilibrium,
floated with ventral sides up and did not respond to touch and they were
promptly removed.
The arithmetic graphic
method was employed in determining the 96-hr LC50. Percentage mortality after 96 hours was calculated and
plotted on the ordinate axis against the pH value on the abscissa. Each point
was then plotted and connected graphically. A horizontal line was drawn from
the 50% survival point to intersect the plot from which point, a vertical line
is dropped to the abscissa. This intersection point on the abscissa
corresponded to the 96 hr LC50.
This was done for all three replicates for each pH value and the mean
determined. Safe pH level used as lower limit for the selected range was
determined using an application factor of 1.03 based on the work of Reish and
Oshida (1986).
Hematological analysis on each treatment were conducted on weekly basis
by sacrificing 2 fish species and blood samples collected using insulin syringe
and needle rinsed with EDTA to determine the various hematological parameters
(Wedemeyer and Yasutake, 1977). The significant differences among means were
tested with 2 –way analysis of variance (ANOVA, 0.05) (Zar, 1984).
Analysis
of physicochemical parameters
Water samples were
analysed regularly to ensure that the expected water quality were maintained.
The analyses for the water quality were conducted using standard procedures as
indicated in APHA (1998)
RESULTS
Physical-chemical
quality of waters
The results of the physico
chemical analysis of the water surface water of the treatment and control tanks
are presented in Table 1.
|
Table 1. The range and mean of the water quality
(physico-chemical) of the treatment and control tanks of water samples in the Niger Delta wetland, Nigeria. |
||||||
|
Treatment |
Feature |
Temperature (oC) |
pH |
Conductivity µS/cm |
Dissolved oxygen (mg/l) |
Biochemical oxygen demand BOD5 (mg/l) |
|
3.6 |
Range |
23.6-24.1 |
3.6 |
36.0-38.0 |
3.0-3.7 |
0.58-0.66 |
|
|
Mean |
23.8 |
3.6 |
37.0 |
3.21 |
0.64 |
|
4.0 |
Range |
23.5-24.2 |
4.0 |
36.0-40.0 |
3.32-3.8 |
0.56-0.64 |
|
|
Mean |
23.7 |
4.0 |
38.2 |
3.4 |
0.62 |
|
5.0 |
Range |
23.4-24.2 |
5.0 |
36.0-38.5 |
3.1-3.8 |
0.59-0.69 |
|
|
Mean |
23.8 |
5.0 |
37.1 |
3.5 |
0.64 |
|
6.0 |
Range |
23.4-24.6 |
6.0 |
36.2-39.3 |
3.1-3.8 |
0.57-0.68 |
|
|
Mean |
23.9 |
6.0 |
37.7 |
3.5 |
0.63 |
|
7.0 |
Range |
23.6-24.3 |
7.0 |
36.6-38.8 |
3.2-3.8 |
0.57-0.66 |
|
|
Mean |
23.8 |
7.0 |
37.7 |
3.5 |
0.63 |
|
Control |
Range |
23.5-24.1 |
6.3-6.5 |
36.8-39.0 |
3.1-3.8 |
0.58-0.71 |
|
|
Mean |
23.8 |
6.4 |
37.2 |
3.5 |
0.65 |
96hr L.C50 test
Data on 96hr exposure of twenty (20) samples
each of S. melanotheron at different
pH levels are presented in Table 2. The test showed increased mortality with increased
acidity. With the arithmetic graphic method, the 96hr LC50 for S. melanotheron was 3.68 (Figure 1).
|
Table 2. Mean mortality
after 96hrs exposure of Seratherodon
melanotheron to different pH in the Niger Delta wetland, Nigeria. |
||||||
|
pH |
Mortality/Duration |
Total mortality |
Percentage mortality |
|||
|
24 hrs |
48 hrs |
72 hrs |
96 hrs |
|||
|
2.5 |
20 |
N.M |
N.M |
N.M |
20 |
100 |
|
3.0 |
8 |
6 |
3 |
1 |
18 |
90 |
|
3.3 |
7 |
4 |
2 |
2 |
15 |
75 |
|
3.6 |
5 |
4 |
2 |
1 |
12 |
60 |
|
4.0 |
N.M |
N.M |
N.M |
N.M |
N.M |
0 |
|
NM: No
mortality |
||||||
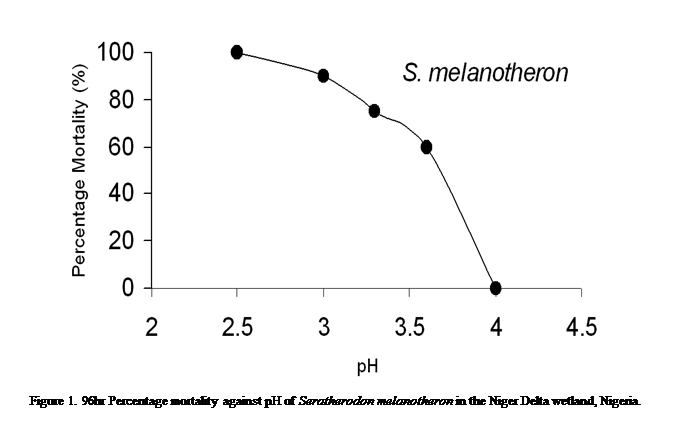
Effect of pH on Sarotherodon
melanotheron
Behavioural Changes
S. melanotheron samples were
observed to exhibit very erratic and disturbed movement, which increased at low
pH levels (Table 3). While the response was immediate at pH 3.8 (commencing in
day 1), it was delayed at pH 4.0 and 5.0, commencing in days 3 and 6
respectively. Between weeks 2 and 3, the intensity of behaviour was reduced
considerably when compared to normal at pH 3.8, 4.0 and 5.0 as was observed in
the control. At weeks 4 and 5 movement was slow and lethargic in fish
maintained at pH 3.8 and 4.0 respectively. Fish maintained at pH 6.0,
7.0, 8.0 and control did not show any abnormal pattern in fish movement.
Neither shoaling nor surfacing for atmospheric air was observed in the
different tanks. Mucus secretion was high at low pH levels of 3.8, 4.0 and 5.0
at weeks 3, 4 and 5 respectively. Secretion was normal in the other tanks
throughout the test period. While mortality exceeded 50% at pH 3.8, and 4.0 at
weeks 5 and 6 respectively, pH 5.0 and 6.0 recorded low mortality rates of less
than 50%. Survival of 100% was observed in fish kept at pH 7.0, 8.0 and control
tanks.
|
Table 3. Behavioural
changes in Sarotherodon melanotheron
at different pH levels in the Niger
Delta wetland, Nigeria. |
|||||||
Indices
|
pH |
||||||
|
3.8 |
4.0 |
5.0 |
6.0 |
7.0 |
8.0 |
Control |
|
|
Movement Intensity/
Onset |
Fast and Very Erratic /Day 1 |
Fast and
Very Erratic /Day 3 |
Fast and
Very Erratic / Day 6 |
Fast and
Erratic /Day 1 |
Fast and
Erratic/ Day 1 |
Fast and
Erratic/ Day 3 |
Fast and
Erratic/ Day 1 |
|
Fast and
erratic/ weeks 2 and 3 |
Fast and
erratic/ weeks 2 and 3 |
Fast and
Erratic/ Week 2 |
|
|
|
|
|
|
Slow and
Lethargic /week 4 |
Slow and
Lethargic/ week 5 |
|
|
|
|
|
|
|
Mucus
Secretion: Intensity/
Onset |
+++/WK3 |
+++/WK4 |
+++/WK5 |
+/WK1 |
+/WK1 |
+/WK1 |
+/WK1 |
|
Mortality |
60% Wk 5 |
55% Wk 6 |
55% Wk 8 |
0 |
0 |
0 |
0 |
|
+++ : High; ++ : Medium; + :
Normal and - : Did not occur |
|||||||
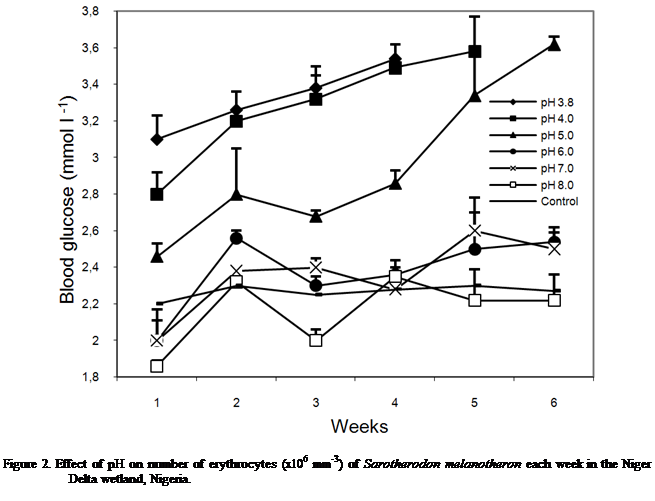
Blood
Glucose
Blood glucose levels increased with acid
level (Figure 2). At pH 3.8 and 4.0 the glucose levels showed exponential
increases over time. At pH 5.0, increase in values with time was also observed
except for a decline in week 3. Similar pattern was exhibited at pH 6.0 though
at lower values. At pH 7.0, the glucose level indicated initial increases to
week 3 but fluctuated thereafter. Glucose level fluctuated at pH 8.0 to week 5
but stabilized at week 6. In fish in the
control tanks (pH), the blood glucose remained relatively uniform value
throughout the experimental period (Figure 2). The values showed significant
differences in the blood glucose between the treatments [F cal = 74.05 > P
(2.60) 0.05] and with exposure time [F cal= 14.50 > P (2.60) 0.05].
Total white
blood cell count
The total white blood cell count of S.
melanotheron at different pH levels
is presented in Figure 3. S. melanotheron
exposed to pH 3.8, 4.0, 5.0 and 6.0
showed a gradual increase in the count over time except for a slight decline at
week 3 for pH 5.0 (Figure 3). At pH 7.0, values were observed to change
marginally throughout the exposure time. At pH 8.0, the white blood cell count
showed more pronounced fluctuation; it declined in week 2, rose in weeks 3 and
then declined steadily through the remaining weeks. Ovoid-shaped leucocytes
with eccentric nuclei were observed under the microscope. Differential count
showed that these cells occurred mostly as lymphocytes and neutrophils.
Monocytes occurred in very low percentages (Table 4).
Statistical
analysis of the changes recorded showed significant differences in the effect
of pH [F cal =21.68 > P (2.60) 0.05].
However there was no statistically significant difference in cell count with
exposure period [F cal = 0.533 < P
(2.60) 0.05].
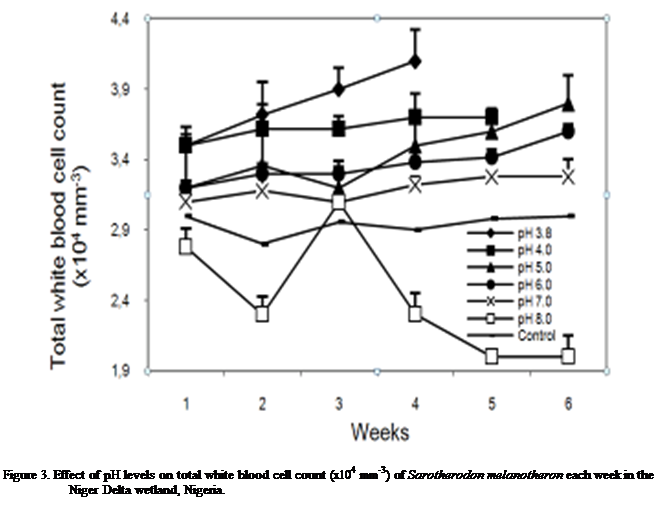
Table 4. Mean
values of differential Leucocyte Count (%) of Sarotherodon melanotheron
exposed to different pH levels and weeks in the Niger Delta wetland, Nigeria.
|
|||||||||
|
Weeks |
1 |
2 |
3 |
||||||
|
pH |
Lym |
Mn |
Nt |
Lym |
Mn |
Nt |
Lym |
Mn |
Nt |
|
3.8 |
77.58 |
2.86 |
19.56 |
75.00 |
2.97 |
22.03 |
76.50 |
3.08 |
20.42 |
|
4.0 |
80.00 |
2.92 |
17.08 |
82.75 |
3.36 |
13.89 |
85.50 |
3.48 |
11.02 |
|
5.0 |
83.00 |
2.92 |
14.08 |
82.18 |
3.40 |
14.42 |
80.95 |
3.62 |
15.43 |
|
6.0 |
83.24 |
2.98 |
13.78 |
81.00 |
3.72 |
15.28 |
82.00 |
3.80 |
14.20 |
|
7.0 |
85.00 |
3.60 |
11.40 |
83.23 |
3.75 |
13.02 |
83.55 |
3.88 |
12.57 |
|
8.0 |
85.00 |
3.50 |
11.50 |
87.10 |
3.75 |
9.15 |
88.20 |
3.84 |
7.96 |
|
Control |
83.50 |
3.50 |
13.00 |
85.00 |
3.50 |
11.50 |
85.00 |
3.00 |
12. 00 |
|
|
|
|
|
|
|
|
|
|
|
|
Weeks |
4 |
5 |
6 |
||||||
|
pH |
Lym |
Mn |
Nt |
Lym |
Mn |
Nt |
Lym |
Mn |
Nt |
|
3.8 |
74.80 |
2.80 |
22.40 |
75.00 |
2.86 |
22.14 |
75.00 |
2.80 |
22.20 |
|
4.0 |
84.00 |
3.60 |
12.40 |
84.00 |
3.10 |
12.90 |
85.37 |
3.00 |
11.63 |
|
5.0 |
80.00 |
3.66 |
16.34 |
81.22 |
3.56 |
15.22 |
81.80 |
3.50 |
14.70 |
|
6.0 |
82.00 |
3.82 |
14.18 |
82.66 |
3.90 |
13.44 |
83.00 |
3.90 |
13.10 |
|
7.0 |
81.60 |
3.84 |
14.56 |
81.00 |
3.76 |
15.24 |
81.80 |
3.90 |
14.30 |
|
8.0 |
85.60 |
3.90 |
10.50 |
85.00 |
3.90 |
11.10 |
87.00 |
3.88 |
9.12 |
|
Control |
88.50 |
2.00 |
9.50 |
84.00 |
2.50 |
13.50 |
85.00 |
3.50 |
11.50 |
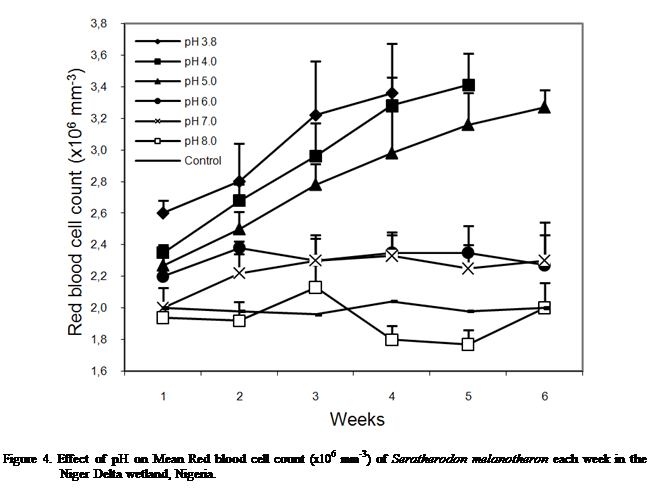
Red blood cell count
The red blood cell count of S.
melanotheron at different pH levels are presented in Figure 4. The changes
at pH 3.8, 4.0 and 5.0 are consistent with those observed with other
parameters; a sharp rise with exposure time, the rise being proportional with
the acid stress (Figure 4).
The changes in red blood cell
count did not appear appreciable at pH 6.0 whereas at pH 7.0, values rose
gradually to week 3 and stabilized in the remaining weeks. At pH 8.0, no definite pattern was observed
in the changes in RBC count. Control pH maintained relatively steady values
throughout the period with nucleated and non-nucleated cells also observed.
Statistical analysis of data
showed that calculated F is greater than the critical F for effects due to both
pH and exposure time. Hence, there is a significant difference in the recorded
changes due to pH effect; [F =29.62 >
P (2.60) 0.05] and exposure time, [F (5.93) > P (2.60) 0.05].
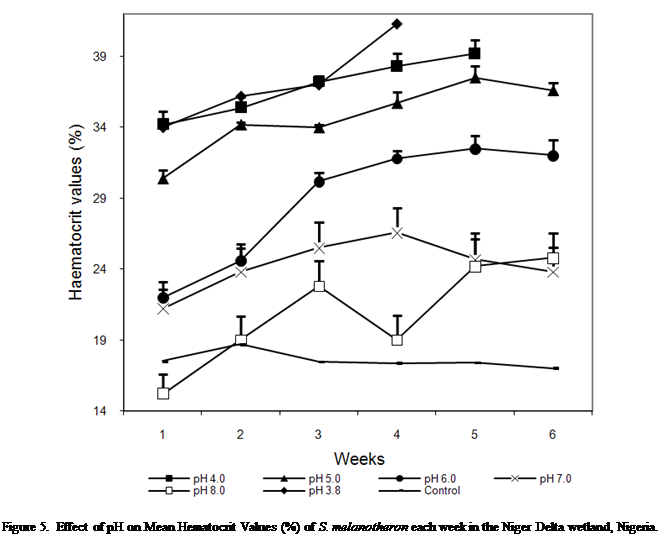
Hematocrit
The hematocrit values of S.
melanotheron at different pH levels are presented in Figure 5. At pH 3.8,
4.0 and 5.0, the hematocrit values increased throughout the experimental period
except for a very slight decline in week 2 at pH 5.0. At pH 6.0 values
increased steeply up to week 3 after which the increases became more gradual.
At pH 7.0, value increased gradually up to week 4 before gradually declining in
the remaining period. Values at pH 8.0 rose up to week 3, declined in week 4
and continued its rise in weeks 5 and 6. Hematocrit values at control pH were
steady throughout the experiment.
DISCUSSION
The physicochemical
parameters of the fish examined in this study showed values characteristic of
freshwater environment. The pH of the surrounding medium was slightly acidic
(6.4) and dissolved oxygen concentration was well as other attributes measured
were adequate to support freshwater aquatic life.
The erratic and abnormal movement of the fish such as regular surfacing at
the water column especially at acidic pH of 3.8 and 4 is evidence discomfort
implying a measure stress on the physiological function of the fish species
which was not observed on fishes exposed to elevated pH (6.0, 7.0 and 8.0). The
importance of this observation is that fishes exposed to low pH conditions
either in the natural habitat or reared in aquaculture pond will suffer similar
stress condition and this may induce growth retardation, reproductive failure
and eventual lead to the mortality of fishes.
In addition, the
progressive increase in values of plasma glucose observed point to the fact
that the fish (S. melanotheron) demonstrated obvious hyperglycemic response
during the exposure to sublethal pH regimes. This signifies that acidic pH
conditions may prevent the complete metabolism of blood sugar to glycogen. This
significant change in blood glucose level with pH suggests a stress response
with tendency of enhancing negative osmoregulatory status in the fish. Wood (1991), environmental acidification from
anthropogenic sources has been identified as a major factor affecting salmonid
populations
Chindah et al. (2004) observed similar
hyperglycaemic response on a common Niger Delta wetland catfish (Clarias
buthopogon). Omoregie et al,
(1990) reported that this incomplete metabolism could induce impaired
osmoregulation. The observed plasma glucose levels in the S. melanotheron are in consistent with the works of Wedemeyer
(1973), Mcleay and Brown (1975), Krishnamurthy et al. (1981), Dheer et al.
(1987), Omoregie et al, (1994) and
Omoregie, (1998). The increased blood glucose level in fishes suggests the
presence of the stress hormones such as catecholamines and corticosteroids, in
the peripheral blood (Fager, 1967; Selye, 1973) and this scenario demands for
increased energy requirement in order for the fish to withstand the acid stress
condition. The secretion of these hormones induces marked changes in
carbohydrate reserves which according to Oguri and Nace (1966) is responsible
for the hyperglycemias. Although glycogen reserves were not monitored, it is
probable that the reported lethargy before death may be associated with
reduction in muscle glycogen (Duncan and Klaverkamp, 1983).
These significant
increases in values for hematological and mucus secretion of the gills
attributes between treatments of the test species (S. melanotheron) are associated with the low acidic
condition. The observed secretion of
mucus by the gills is an evidence suggesting irritation due to stress
conditions (Omoregie et al, 1994 and
Omoregie, 1998). This mucus cover of the gill surface may possibly impair its
functions in oxygen exchange. This
development, could lead to dehydration and enhance reduction in the blood
oxygen level to which the fish homeostatic system responded to by the observed
increases in the erythrocytes, lymphocytes and hematocrit levels in order to
increase the efficiency of transporting the reduced oxygen in the blood. This
observed increase in erythrocytes,
lymphocytes and hematocrit levels contrasted with the works of Sikoki et al. (1989), Omoregie et al. (1990) and Omoregie et al. (1994) all of whom reported
decreases in values of these parameters in juveniles of Clarias garienpinus and Oreochromis
niloticus when exposed to sublethal concentrations of other stress factors
(heavy metals, crude oil and formalin). However, our result is in consonance
with those of Vaala and Mitchell (1970) and Vaala (1972), which independently
reported that fish subjected to acid stress, may experience a decrease in
arterial oxygen level and respond to this hypoxemia by increasing the
oxygen-carrying capacity of the circulating blood. This development is
manifested in those parameters associated with oxygen transport – erythrocytes,
hematocrit and hemoglobin (Neville, 1979; Spry et al. 1981; Milligan and Wood, 1982). Wedemeyer and Mcleay (1981)
also reported that the high values of erythrocytes, leucocytes and hematocrit
indicate hemoconcentration possibly due to gill damage and dehydration.
The more active nature
of S. melanotheron, depicts its hematological
requirements of high oxygen demand to meet the requirements of a high metabolic
rate, hence the significantly higher hemoglobin and hematocrit values at acid
stress levels reported for S. melanotheron in this study. The high values recorded for these parameters in S. melanotheron may also be due to their blood rich
gills exposed almost directly to the oxygen in the water column thus limiting
the effect of unfavourable aquatic pH on respiration and energy demand. This is
consistent with earlier observations in comparative hematology (Engel and
Davis, 1964; Larsson et al,
1976). Mavares and Perez (1984), Rambhaskar and Srinivasa (1986) and Chindah et al. (2000) also reported that active fish also
have higher values of erythrocyte in addition to high hematocrit and hemoglobin
levels.
Mature red blood cells are usually nucleated. The
observation that non-nucleated cells were also seen indicate that fishes
respond to maintain homeostasis in the peripheral blood cell population by
facilitating the quick transfer into the blood stream, of non-nucleated red
blood cells which occur in their penultimate stage of development. The observed
mean RBC of 1.99 x 106mm-3 at control pH for S.
melanotheron is higher than values reported by Etim et al. (1994) in similar studies for Chrysichthys nigrodigitatus
(1.77 x 106mm-3), Chrysichthys furcatus (1.98 x 106mm-3),
Ictalurus nebulosus (1.2 x 106mm-3), and Ictalurus
punctatus (2.16x106mm-3).
The hematocrit and hemoglobin values at their
control pH were recorded as 17.8% and 6.3g/dl for S. melanotheron. These
values support results of earlier studies by Clark et al. cited by Oranye, (2002) that reported fish hematocrit values
of between 20-35% scarcely attaining values higher than 50% while Larsson et al. (1976) actually reported
hematocrit values of 51.3% and 52.3% for Clupea harengus and Scomber
scrombrus respectively and hemoglobin values of 14.0g/dl and 12.7g/dl.
The results of leucocyte counts (2.94
x104 mm-3) are lower than values reported for C. nigrodigitatus and C.
furcatus (5.82 x 104 mm-3 and 3.1 x 104 mm-3)
respectively (Etim et al,1994). The increase in leucocyte counts with time in both
species depicts an attempt at enhancing the body’s defense mechanism arising
from increasing stress levels. This appears also to be associated with the
observed high mucus secretion at stress levels indicative of disease condition.
It is worthy of note that the changes in the
leucocyte counts for the fish species points to the occurrence of lymphocytes,
monocytes and neutrophils. Thrombocytes, known to be the critical cells
involved in fish blood coagulation, as with other vertebrates, were not
detected, yet the rapidity with which blood clotted during the sampling
procedure when insufficient anti-coagulant was used indicated substantial
presence of these cells. It is probable that failure to detect these cells is a
reflection of an increase in their fragility such that when a blood smear is
prepared; the cytoplasm is stripped away leaving denuded nuclei which often
appear as lymphocytes. Ellis (1977) argued that only occasionally can the
entire thrombocyte population appear as undisrupted cells and be differentiated
from lymphocytes. A more accurate determination of thromobocytes population may
be done using the immuno-fluorescent technique which stains only the
lymphocytes. The number of lymphocytes in fish can vary widely between
individuals of even a single species. Nonetheless, the very high percentage of
lymphocytes recorded in this study alongside the fact that thrombocytic cells
were not seen seems to indicate that the thrombocytes must have appeared as
lymphocytes as reported by Ellis (1977).
It is therefore concluded that S. melanotheron
responded negatively to low acidic levels which generates unfavourable
physiological conditions affecting body fluids, physiological functioning of
the body, and perhaps may degenerate further to cause reproductive failure and
mortality.
LITERATURE CITED
American Public Health Association (Apha). 1998. Standard Methods for the
Examination of Water and Waste water. p.
16 and 75-427.
Akiri, P. J.
1987. Taxonomy of the Genus Clarias (Pisces: Siluriformes) in Rivers
State, Nigeria and the Ecology of its species in relation to selected
freshwater habitats. Ph. D Thesis. Rivers State University of Science and
Technology (Department of Biological Science). p. 33, 67 and 145.
Brown, D. J. A.; R.
Morris and S. A. Goldthorpe. 1984. Sublethal effects of acid water. In Stress and Fish. A. D. Pickering (ed.).
Academic Press. London. U. K. 562 p.
Casillas, E. and L. S.
Smith. 1977. Effect of stress on blood coagulation and haematology in rainbow
trout (Salmo gairdneri). Journal of
Fish Biology 10 (5): 481-491.
Chindah, A. C.; F. D.
Sikoki and I. Vincent-Akpu. 2000. Toxicity of Cypermethrin to Tilapia guineensis Jurveniles. J. Agric.
Biotech. Environ. 2 (1&2): 60-66.
Chindah, A. C.; S. A.
Braide and R. O. Oranye. 2004. Response of a common Niger Delta Wetland Catfish
to changes in pH. Niger Delta Biologia 4 (2): 56-65.
Chindah, A. C. and A.
I. Hart. 2000. Occurrence and distribution of epifauna and infauna community in
shallow mangrove wet land in the tropical West African Region. Afri. J.
Environmental Studies. 1: (1&2): 76-83.
Dheer, J. M. S.; T. R.
Dheer and C. L. Mahajan. 1987. Haematological and haematopoietic response to
acid stress in an air-breathing freshwater fish, Channa punctatus Bloch. Journal of Fish Biology 30 (5): 577-588.
Duncan, D. A. and J.
F. Klaverkamp. 1983. Tolerance and
resistance to cadmium in white suckers, Catastomas
commersani previously exposed to cadmium, mercury, zinc and selenium. Canadian Journal of Fisheries and Aquatic
Science. 40: 128-138.
Ellis, A. E. 1977. The
leucocytes of fish: A review. Journal of Fish Biology 14: 453-491.
Engel, D. M. and E. M.
Davis. 1964. Relationship between activity and blood composition in certain
marine teleosts. Copeia 1964 (3):
586-587.
Etim, L.; S. B. Ekanem and A. Utin. 1994. Haematological profile of 2 species of
Catfish, Chrysichthys nigroditalus
Lacepede and Chrysichthys furcatus Gunther
from the great Kwa Rivers, Nigeria. Global Journal of Pure and Applied Science 5 (1): 262-268.
Fager, U. H. M. 1967. Plasma cortisol concentration
in relation to stress in adult Sockeye Salmon during freshwater stage of life
cycle. Gen. Comp. Endocrin. 8: 197-200.
FAO Fisheries Report No. 587. 1997. Working party on
pollution and fisheries committee for inland fisheries of Africa. p. 7, 9 and
10.
Ibiebele, D. D. 1987. Oshika Oil Spill incident. A
case study four years after spill. Proceedings of the Petroleum Industry and
the Nigerian Environment.
Krishnamurthy, V.; P. Reddanna and S. Govindappa.
1981. Hepatic carbohydrate metabolism in Tilapia
mossambica (Peters) acclimated to low environmental pH. Can. J. Zool. 59: 400-402.
Larsson, A.; M. L. Johansson-Sjabeck and R. Fange.
1976. Comparative study of some haematological and biochemical blood parameters
in fishes from Skagerak. Journal of Fish Biology 9: 425-430.
Mavares, R. N. and J. E. Perez. 1984. Blood
adaptations to marine and fresh water environments in fish of the family
Sciaenidae (Perciformes). Journal of Fish Biology 25: 657-659.
McLeay, D. J. and D. A. Brown. 1975. Effects of
acute exposure to bleached Kraft pulpmill effluent on carbohydrate metabolism
of juvenile Coho salmon (Oncorhynchus
kisutch) during rest and exercise. Journal of the Fisheries Research Board
of Canada 32:753-760.
Milligan, C. L. and C. M. Wood. 1981. Branchial and
renal acid and ion fluxes in the rainbow trout, Salmo gairdneri, at low environmental pH. J. Exp. Biol. 93: 101-107.
Neville, C. M. 1979. Sublethal effects of
environmental acidification on rainbow trout, Salmo gairdneri. Journal
of the Fisheries Research Board of Canada 36: 84-85.
Oguri, M. and P. F. Nace. 1966. Blood sugar and
adrenal histology of the gold fish after treatment with mammalian
adreno-corticotrophic hormone.
Chesaspeake Science 9:
198-199.
Omoregie, E.; E. B. C. Ufodike and I. R. Keke. 1990.
Tissue chemistry of Oreochromis niloticus
exposed to sublethal concentrations of gammalin 20 Actellic 25 EC. J. Aquatic Science 5: 33-36.
Omoregie, E.; T. G. Eseyin and P. C. Ofojekwu. 1994.
Chronic effects of formalin on erythrocyte counts and plasma glucose of Nile
tilapia Oreochromis niloticus. Asian
Fisheries Science 7: 1-6.
Omoregie, E. 1998. Changes in the haematology of the
Nile tilapia Oreochromis niloticus
Trewavas under the effect of crude oil. J. Acta Hydrobiologica. 4: 287-392.
Oranye, R. O. 2002. Haematological responses to acid
stress in Clarias buthopogon and Sarotherodon melanotheron. M.Phil
Thesis, Institute of Geoscience and Space technology Rivers State University of
Science and Technology Port Harcourt. I-XI. 287 p.
Pudo, J.; A. Lysak and J. F. Afred-Ockiya. 1990. The
interrelationship of phytoplankton and fish species in tropical brackish water
fish ponds of Southern Nigeria. Acta Hydrobiol. 32(1/2): 227-235.
Rambhaskar, B. and R. K. Srinivasa. 1986.
Comparative haematology of ten species of marine fish form Visakhapatnam Coast.
J. Fish Biol. 30: 59-62.
Reish, D. J and P. S. Oshida. 1986. Manual of
methods in aquatic environment research. Part 10. Short term static bioassays.
FAO Fish Technical Paper (247) 22 p.
Sadler, K. and S. Lynam. 1987. Some effects on the
growth of brown trout from exposure to aluminum at different pH levels. Journal
of Fish Biology 31: 209-219.
Selye, H. 1973. The evolution of the stress
concept. Am. Science. 61: 692- 699.
Kori-Siakpere, O. 1985. Haematological
characteristics of Clarias isheriensis Sydenham. Journal of Fish
Biology 27 (3): 259-263.
Sikoki, F. D.; A. I. Ciroma and C. Ejike. 1989.
Haematological changes in Clarias
geriepinus following exposure to sublethal concentrations of zinc, lead and
cadmium. In: Onyia, A.D. and G.N. Asala (Eds.). Proceedings of the 7th
Annual Conference of Fisheries Society of Nigeria, FISON, Bukuru, Jos, Nigeria.
p: 20-26.
Spiff, A. I. and M. N. Horsefall. 1998. Principles
of environmental chemistry. Metroprints Ltd. Port Harcourt. 82 p.
Spry, D. J.; C. M. Wood and P. V. Hodson. 1981. The
effects of environmental acid on freshwater fish with particular reference to
the soft water lakes in Ontario and the modifying effects of heavy metals. A
literature review. Can. Tech. Rep. Fish. Aquat. Sci. 999: 144 pp.
Trewavas, E. 1983. Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia.
London.
Vaala, S. S. and R. B. Mitchell. 1970. Blood oxygen
tension changes in acid exposed brook trout.
Proceedings of Pennsylvania Academy of Science 44: 41- 44.
Vaala, S. S. 1972. Erythrocytic indices of stress in
brook trout (Salvelinus fontinalis)
exposed to sublethal levels of acidity. Proceedings of Pennsylvania Academy of
Science 45: 110-112.
Wedemeyer, G. A. 1973. Some physiological aspects of
sublethal heat stress in the juvenile steelhead trout (Salmo gairdneri) and Coho salmon (Oncorhynchus kisutch) Journal
of the Fisheries Research Board of Canada 30: 831-834.
Wedemeyer, G. A. and W. T. Yasutake. 1977. Clinical
methods for the assessment of the effects of environmental stress on fish
health. Technical Paper of the U.S. Fish and Wildlife Service. Washington D. C. 89. p. 19-21.
Wedemeyer, G. A. and D. J. Mcleay. 1981. Methods for
determining the tolerance of fishes to environmental stressors: In Stress
and Fish. A. D. Pickering (ed). Academic Press. London, U. K. p. 247-275.
Wood, C. M. 1991. Acid-base and ion balance,
metabolism and their interactions after exhaustive exercise in fish. J. Exp.
Biol. 160: 285-308.
Zar, H. J. 1984. Biostatistical analysis. 2nd
Edition. Prentice Hall, England
Cliffs. p. 328 and 334.
Página diseñada por Prof. Jesús Rafael Méndez Natera
TABLA DE CONTENIDO DE LA REVISTA CIENTÍFICA UDO
AGRÍCOLA


