Revista Científica UDO Agrícola Volumen 7. Número 1. Año 2007. Páginas: 258-273
Succession of phytoplankton in a municipal waste water
treatment system under sunlight
Sucesión del fitoplancton en un medio de tratamiento
de aguas provenientes de desechos Municipales utilizando luz solar
Alex Chuks CHINDAH ![]() 1, Solomon Amabaraye BRAIDE1,
Jonathan AMAKIRI2 and Ebele IZUNDU1
1, Solomon Amabaraye BRAIDE1,
Jonathan AMAKIRI2 and Ebele IZUNDU1
1Institute
of Pollution Studies. Rivers State University of
Science and Technology. Nkpolu Oroworukwo. P. M. B. 5080, Port Harcourt. Rivers State, Nigeria and 2Plant Science and
Biotechnology. University of Port Harcourt, Port Harcourt,
Nigeria. E-mails: alexchindah@yahoo.com and
alexchindah@hotmail.co.uk ![]() Corresponding author
Corresponding author
|
Received:
08/21/2007 |
First reviewing ending: 10/09/2007 |
First review received: 10/29/2007 |
|
Second reviewing ending: 11/26/2007 |
Second review received: 12/07/2007 |
Accepted:
12/13/2007 |
Abstract
A study on succession of
phytoplankton in a municipal waste water treatment was conducted from a major
drainage stream system receiving municipal wastes from densely populated urban
municipality of Port Harcourt in Rivers State, Niger Delta of Nigeria. The
study area lies within 4º 88′′ - 4º 99′′ N and 5º 00′′
- 5º 14′′ E. The study was carried out to investigate the variation
of phytoplankton communities and their interactions with the physico-chemical characteristics of the waste water being
treated with sunlight. The phytoplankton population indicated six (6) major
successional development patterns in the recruitment of species. This affected
the distribution of phytoplankton descriptors such as species diversity -H’
(that decreased from 0.99 on the 1st day to 0.62 on the16th day),
dominant index -DI (with minimum of 0.000085 on the 1st day to
maximum of 0.12 on the 11th day), community structure and biomass at
various stages of the depuration and correlated differently with the
physicochemical parameters. The results suggest that phytoplankton was mainly
regulated by nutrients and the massive Cyanobacterial
bloom declined as the water quality improved which were well related to changes
in algae diversity, dominance index, abundance and biomass. A model to compare
actual and predicted values indicated some coherence between several biological
and physicochemical attributes.
Kew
words:
Phytoplankton, municipal wastewater, dominant index, Cyanobacteria
RESUMEN
Se condujo un estudio sobre la sucesión del fitoplancton en un
tratamiento de aguas residuales Municipales en muestras de un sistema
importante de corrientes de drenaje, el cual recibía desechos municipales de un
área urbana densamente poblada de Diobu en la
Municipalidad de Port Harcourt, Rivers
State, Niger Delta de
Nigeria. El área de estudio se encuentra entre la longitud 4º 99’’ y 4º 88’’ N
y la latitud 5º 00’’ y 5º 14’’ Este. El experimento fue realizado para
investigar la variación de las comunidades del fitoplancton y su interacción
con las características físico-químicas de las aguas residuales las cuales se
trataron con luz solar. La población del fitoplancton indicó seis patrones
principales de desarrollo sucesional en el
reclutamiento de las especies. Esto afectó la distribución de los descriptores
del fitoplancton tales como la diversidad de especies -H’ (que disminuyó de
0,99 en el primer día a 0,62 en el día 16), el índice de dominancia –ID (con un
valor mínimo de 0,000085 en el primer día a un valor máximo de 0,12 en el
undécimo día), la estructura de la comunidad y la biomasa en diferentes etapas
de la depuración y correlacionó de manera diferente con los atributos
físico-químicos. Los resultados sugieren que el fitoplancton estuvo
principalmente regulado por los nutrimentos y la floración masiva de la Cianobacteria disminuyó a medida que mejoró la calidad del
agua, las cuales estuvieron bien relacionadas con los cambios en la diversidad
de las algas, el índice de dominancia, la abundancia y la biomasa. Un modelo
para comparar los valores reales y predichos indicó alguna coherencia entre
varios atributos biológicos y físico-químicos.
Palabras claves: Fitoplancton, aguas residuales municipales, índice de dominancia, Cianobacteria
Introduction
The
concern on the quantity and quality of waste generated and discharged into
natural water bodies has recently indicated the need for different strategies
to address water quality challenges in the regions. The municipal areas of Port
Harcourt were found to have poor water
quality; while water qualities in the outskirts of the cities were considered
fair (Ogan, 1988), associated with dense populations,
and intense economic activity.
This concern on waste water
quality resulted in considering the possible treatment option bearing in mind
inexpensive ways of administering wastes in the third world. The wastes generated and discharged are
mostly from domestic sources (household facilities, open markets, garages
laundry) and small scale industrial set ups (laundry and photographic shops).
These wastes are generally discharged into a nearby water body.
The
population of the area adjoining the study stream system is high (70.25million)
and over 85% of the stream bank is developed with infrastructural facilities
such as concrete residential housing units, garages, photographic shops, car
wash and market stalls. These adjoining activities from these introduce
considerable solid and liquid wastes that impact on the water quality integrity
as it receives about 4500 litres/day of waste
containing petroleum product especially as crankcase oil and spent oil, over
250,000 litres/day of domestic wastes, human wastes
of 120 litres/day, 20kg/day of metal and 58 kg/day of
solid waste such as paper, polyethylene bags and cotton materials (Ogamba, 2003).
These
wastes generated and discharged contain several chemical components that are organic
and inorganic in origin. The impacts of the waste components in altering
habitat integrity of natural water bodies have been reported in previous
studies (Ajayi and Osibanjo,
1981; Ibiebele et
al., 1987; Powell, 1987; Ekweozor et al., 1987; Chindah,
1998; Chindah et
al., 2005). These discharges cause
damage to human health, fisheries, and agriculture, and results in associated
health and economic costs (Okpokwasili and Nwabuzor, 1988; IPS, 1990; Okpokwasili
and Olisa, 1991; Joiris and Azokwu, 1999; Chindah and Sibeudu, 2003, Ndiokwere, 1984).
It also threatens ecosystems through eutrophication, and is responsible for the
loss of plant and animal species. Improving the surface water quality and
sanitation will substantially reduce the incidence and severity of water borne
associated diseases in the area. In developing nations the challenges of
handling and treating waste water has been difficult to due to the unaffordable
financial implication for government to undertake. It is for this reason that research
effort was made to adopt an inexpensive procedure (exposing waste water under
solar radiation) that indicated a measurable success in the physicochemical
quality (Chindah et al., 2005). Understanding
of the dynamics of the biological organism particularly the primary producer in
the treatment system is considered important as information in this respect is
lacking. On the basis of existing gap in knowledge this study was undertaken to
evaluate the response of phytoplankton to the treatment process of municipal
wastes.
Materials and methods
Site description
The
study area has the characteristic feature of tropical equatorial latitude with
high humid and temperature which is
more or less uniform all through the year. Rainfall occurs almost all the
months (May - November) of the year with short duration of dry season (December
- April). The annual average rainfall is 2360mm (Gobo, 1988) and humidity is
generally high for both wet and dry season (> 85%). The natural drainage
basin is largely exposed as vegetation is virtually removed by adjacent
development and macrophytes such as Nymphaea micrantha, N.
lotus, Pistia stratiotes, Ludwigia leptocarpa, Ipomea aquatica, Neptuna oleracea, Cyperus distans, are the only
plants that occupy the outer margin of the drainage system.
Sampling
Samples
for the study were collected from a major drainage basin receiving municipal
wastes from densely populated urban area of Diobu in
Port Harcourt municipality. The area lies within 4o
Physicochemical
parameters
Samples
were collected in one litre plastic containers at sub-surface
level and analyzed in the Institute of Pollution Studies (IPS) laboratory using
procedures as outlined in standard method for the examination of water and
waste water (APHA, 1998). The following parameters were investigated:
temperature, pH, conductivity, alkalinity Dissolved oxygen (DO), Biochemical
oxygen demand (BOD5), ammonia-nitrogen, nitrate-nitrogen, sulphate, phosphate. Temperature was measurement in-situ
using mercury in bulb thermometer. pH, conductivity, turbidity total dissolved
solids (TDS) were measured using multiprobe Horiba instrument (water checker model U-10).
Dissolved oxygen (DO) and Biochemical oxygen demand (BOD5) were
determined using Winkler’s method (APHA,1998). Total suspended solids and
Nutrient parameters (nitrate (NO3-), ammonia (NH3-),
phosphate (PO4-3), sulphate (SO4-2)
were determine using the spectrophotometric method (spectronic
instrument 21D) at various wavelengths based on Standard methods for the
determination of water and wastewater as stated in APHA (1998).
Qualitative and quantitative analysis of Phytoplankton
Phytoplankton and chlorophyll ‘a’ samples were
collected each day in triplicate with 50ml and 20 ml bottles respectively. The
50ml sample was immediately fixed with Lugol’s
solution, allowed to settle for 24hrs before decanting to a uniform
concentration (10ml). The samples were properly homogenized and 1ml sub-sample
from original stock was collected with a sample pipette for numerical analysis.
The pipette content was transferred into a Sedgewick
- Rafter counting chamber for enumeration using a Lietz
binocular microscope magnification of 200x and identification of 1000x using
the reports of Mills (1932), Sieminska (1964), Starmach (1966), Patrick and Reimer (1966) Durand and
Leveque (1980) and Chindah and Pudo
(1991).
Samples for chlorophyll “a” were analyzed using the
trichromatic method as stated in APHA (1998). Twenty ml samples were filtered
through a Whatman membrane filter (0.45µm) and
immediately placed in a vial containing 90% acetone wrapped with foil for chlorophyll
“a” analysis. The filtrate was ground,
centrifuged and the supernatant and blank (acetone) were determined at 663nm and 665nm
wavelength using Spectronic 21D and values obtained
calculated for chlorophyll ‘a’ as indicated in APHA (1998).
Statistical analysis
The
Shannon –Weaver, species diversity index, H′ (Margalef,
1958) was used:
![]()
Where ni is the number of species in group (i), N is the total
number of individuals in (i) group.
Dominance
index
The
dominance index was calculated using the Bergen-Parker dominance index (Chellappa 1990):
D = n
max/NT
Where:
n max = number of individuals of the dominant species.
NT =
total number of individuals of all the species recorded
The
two indices, relationships between physico-chemical
and phytoplankton variables were estimated by simple linear correlation and
regression model analyses performed with Microsoft Excel 2003.
RESULTS
Physicochemical parameters
The
physicochemical changes observed during the treatment process have been
reported earlier Chindah et al., (2005) with the synopsis on the recovery presented in Table
1.
|
Table 1. Physicochemical
variables in the wastewater treatment system from Diobu in Port
Harcourt, Nigeria. |
|||
|
S/no parameter |
Range |
Mean and SD |
% recovery |
|
Temperature (ºC) |
26.5 - 32 |
29.24 ± 2.16 |
ND |
|
pH |
7.2 – 9.0 |
7.91 ± 0.50 |
80,00* |
|
Conductivity (µScm-1) |
506 - 706 |
620.87 ± 70.26 |
72.94 |
|
Turbidity
(NTU) |
3 - 62 |
22.67 ± 13.36 |
95.20 |
|
TDS (mg/l) |
358 - 494 |
440.2 ± 45.81 |
27.10 |
|
TSS (mg/l) |
1.74 - 3.19 |
2.746 ± 0.52 |
45.50 |
|
DO (mg/l) |
0.23 -6 |
2.01 ± 2.15 |
96.00 |
|
BOD5 (mg/l) |
0.92 - 28.5 |
16.25 ±11.86 |
96.80 |
|
COD (mg/l) |
0.81 - 19.95 |
11.38 ± 8.31 |
96.80 |
|
Nitrate
(mg/l) |
0.04 - 0.64 |
0.22 ±
0.16 |
93.75 |
|
Phosphate
(mg/l) |
0.39 - 4.54 |
2.83 ± 1.36 |
91.40 |
|
Sulphate
(mg/l) |
8.81 - 16.01 |
12.46 ±2.82 |
45.90 |
|
ND – not determined, *
increased value |
|||
Phytoplankton
Species occurrence and successional patterns
A
total of 50 phytoplankton species were observed during the study and the taxa
representing four major algal groups (Bacillariophyceae,
Chlorophyceae, Cyanophyceae,
and Euglenophyceae) are contained in Table 2.
|
Table 2. Identified phytoplankton species and their
occurrence in the treatment from urban area of Diobu in
Port Harcourt Municipality, Rivers State, Niger Delta of Nigeria. |
|
|
Cyanophyceae Chrococcus minuta Skuja Chrococcus turgidus
Nag. Chroococcus
sp. Oscillatoria terebriformis Gomont Oscillatoria sp. Merismopedia punctata Meyer Lygbya
pseudospirulina (Utermorhl)Pascher Rhabdoderma
lineare Schm. Lauter. Romeria
elegans (Wolosz.) Kocz. Anacystis aeruginosa Kutz. Anabaenopsis
arnoldis Aptekarj Anabaena
flos-aquae (Lyng.) Breb Gomphosphaeria
sp. Gloeocapsa
magma (Breb) Hullerb. Chlorophyceae Chlamydomonas
spp. Chloromonas
ulla (Skuja) Gerloff et Ettl. Phacotus
laticularis(Ehrenberg) Stein Euastropsis
richter (Schmidle) Lagerheim. Coelastella
levicostata Chodat Tetradesmus
crocici Fott et Kom Scenedesmus
quadricauda (Turpin) Brébisson Euastropsis
spp. Scenedesmus
ecornis (Ehr.) Scenedesmus ovalternus
(Bernard) Chodat Scenedesmus obliguues (Breb.)
Playfair |
Chlorophyceae Scenedesmus pseudodenticulatus Hegewald Ulothrix limeatica
Lemru Roya cambria
W.west & G.E.West Closterium limneticum
Ehr. Cosmarium pyramidatum
Breb. Staurastrum apiculatum
Breb Euglenophyceae Euglena
acus Ehr. Euglena
pascherii Swirenko Lepocinclis
teres ((Schm.tz) Fr. Lepocinclis
stenii Lemm. Phacus
granum Drezepolski Phacus acuminatus
Stokes
Phacus pleuronectes (Ehr.)
Duj. Trachelomonas zuberi
Koczwara Bacillariophyceae Achnanthes lanceolata
(Breb.) Grun. Achnanthes linearis
(W.Sm.) Grun. Fragilaria crotonensis Kitt. Navicula cuspidata Kutz. Navicula minuscula Grun. Navicula minima Grun. Navicula laterostrata Hust. Gomphonema spp. Synedra acus Kutz. Nitzschia
linearis W.Sm. Grun. Pinnularia maior (Kutz) Cl. |
Phytoplankton
species demonstrated variation in occurrence at different times during the
treatment process. It was observed that initial resident species in the phytoplankton
community in the municipal waste water is Merismopedia
punctata,
Anacystis aeuroginosa,
and Romeria elegans,
with the debut emergence of new organisms few days after (Lyngbya
pseudospirulina, Anabaenopsis
arnoldis, Gomphosphaeria sp., Ulothrix
limeatica Lemmru, while other entrants
into the community appear towards the end of the study (Oscillatoria
terebriformis, Gloeocapsa maya, Scenedesmus obligues) (Figure 2).
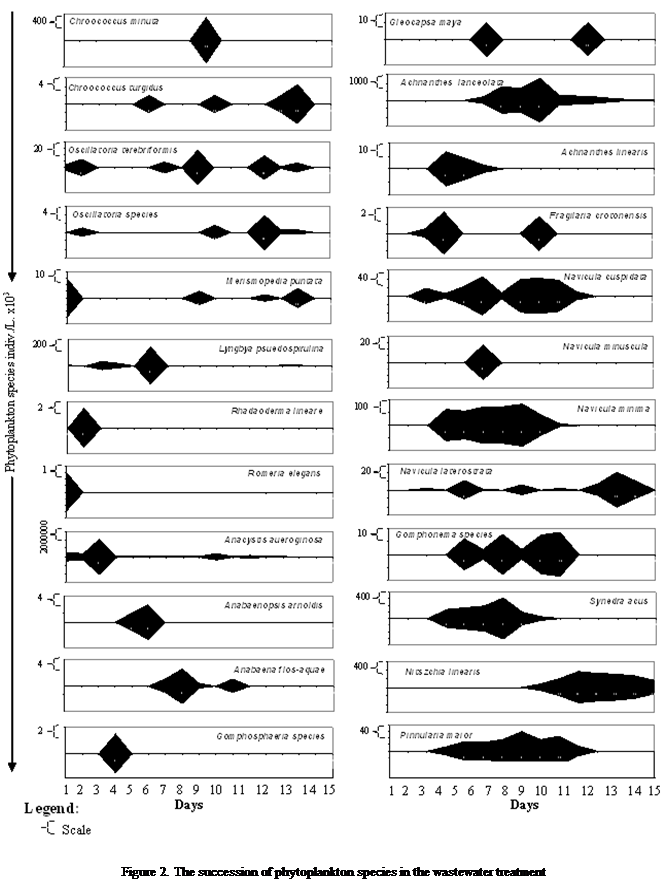
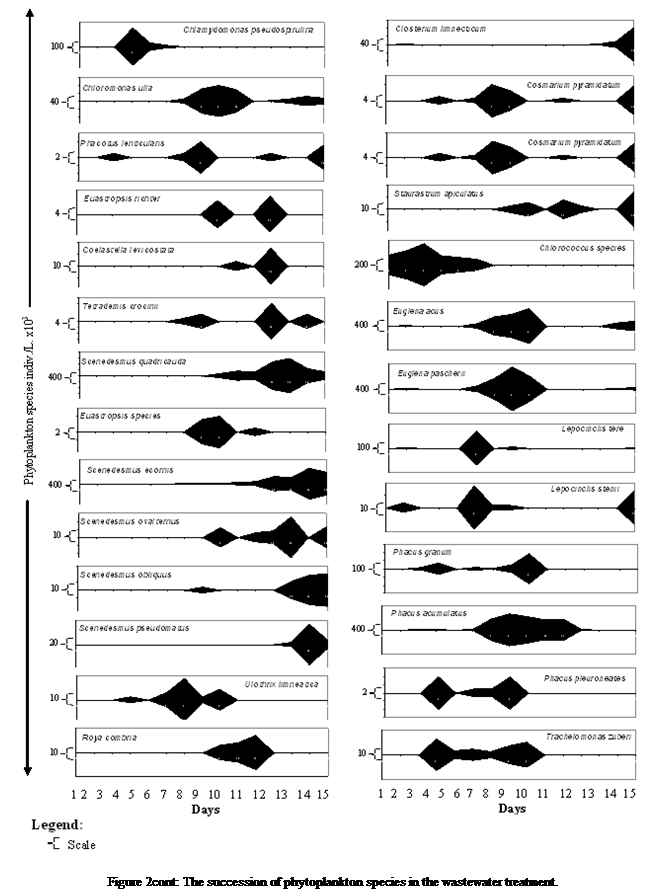
During
the emergence of the species two main characteristic attributes were observed,
firstly were species that erupted and quickly created an outburst in population
(Anabaena flos-aquae, Phormidium
acumulatus, and Navicula
minima), and secondly those that made appearances with negligible impact on
the population density (Rhabdoderma lineare,
and Romeria elegans).
Another
feature observed amongst the species were on status of their residents in the
community, with some species being permanent residence (Anacystis aeuroginosa ) and transitory species that had two suites such
as Euglena pascherii,
Phormidium acumulatus, Achnanthes lanceolata, Navicula minima, and Synedra acus
that occurred earlier during the treatment process and Scenedesmus acornis, Scenedesmus
quadricauda and Nitzschia
linearis that
were observed almost towards the end of the treatment process (Figure 2).
These
two prominent scenarios gave rise to six major successional patterns observed;
firstly was within the 2nd day of
the experiment when Oscillatoria terebriformis, Merismopedia punctata, Romeria elegans, Anacystis aeuroginosa, and Chroococcus species were observed in the community.
The
second pattern was observed a few days later (day 2 - day 4) with species such
as Lepocinclis stenii, Oscillatoria spp., Rhabdoderma lineare,
Lyngbya pseudospirulina, Closterium limneticum, Navicula laterostrata, Navicula cuspidata, Euastropsis richter, Phacotus lendneris, Euglena acus, E. pascherii, Lepocinclis teres (schm), and
Synedra acus predominated
the phytoplankton community. The third
was observed towards the first half (day 5 and day 6) with 13 other species
emerging and contributing to the phytoplankton population (Anabaenopsis arnoldis, Gomphosphaeria
sp., Chlamydomonas
sp., Ulothrix
limeatica, Cosmarium pyramidatum, Fragilaria crotonensis, Phacus pleuroneates, Trachelomonas zuberi, Navicula minima, Gomphonema sp., Pinnularia maior, Phacus granum, and Achnathes lanceolata).
The
fourth successsional pattern occurred midway to the
end of the study (7 – 8th
day) with 10 species (Chroococcus turgdus, Anabaena flos-aquae, Gloeocapsa magma, Chloromonas
ulla, Tetradesmus crocinii, Scenedesmus acornis, Staurastrum apicultus, Scenedesmus obligues, Navicula minuscula, Phacus acumulatus) (Figure 2).
The
fifth was observed close to the ending of the study (9 – 10th day)
with 8 species (Chroococcus minuta, Euastropsis richter, Scenedesmus quadricauda, Euastropis richerii, Coelastrella levicostata,
Scenedesmus ovalternus, Roya cambria, Nitzschia
linearis).
The
sixth pattern was the predominance of Scenedesmus pseudodenticulatus almost at the end of the
experiment (Figure 2).
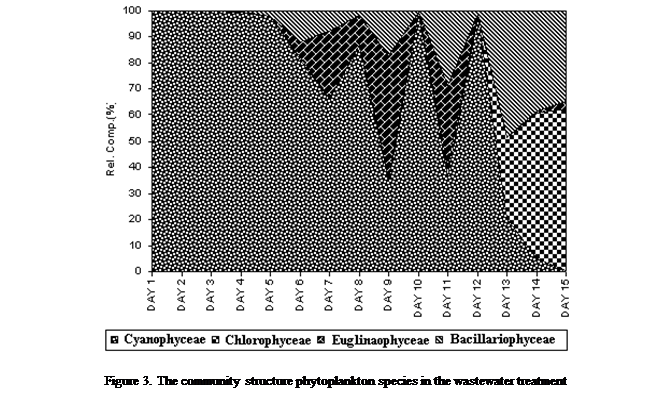
The
community structure initially exhibited preponderance of Cyanophyceae
(blue-green algae) for the first 8 days of exposure (1- 8 days) thereafter
decreased considerably; except on the 10th day and 12th
day, when sudden increase was observed. Other groups in the phytoplankton
community resurgence in proportion over time include, Euglenophyceae,
the first to quickly attain a relatively high importance in the community -
25.6% (day 7) (Figure 3). The concentration increased to a maximum of 47.1% two
days after (day 9) and declined somewhat till the end of the experiment. To the
contrary, Chlorophyceae increased almost steadily
(exponentially) to attain maximum importance in the community at the end of the
experiment (15th day). Similarly, maximum importance by Bacillariophyceae was equally observed in the community
toward the end of the experiment (day 13).
The
species diversity during the depuration process rapidly cascaded from start to
end of the study as maximum species diversity was observed at day 1(H' = 0.99),
diversity values were maintained till the 4th day (H' = 0.99) before declining
steadily to day 7 (0.66) thereafter values oscillated to the end of the
experiment (H' = 0.62), however, the minimum species diversity was on the 11th
day of exposure (Figure 4).
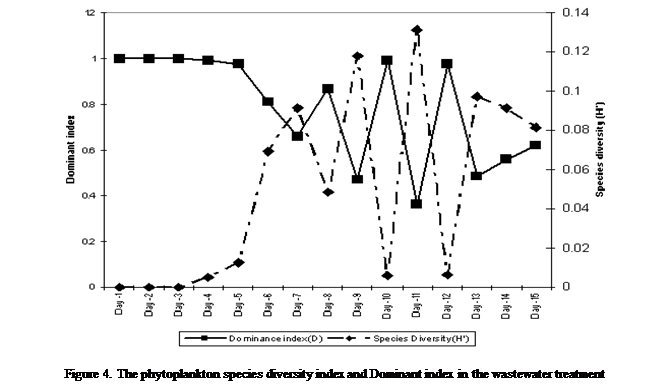
Conversely,
the dominant index had maximum value of 0.12 on the 11th day of the exposure
period and the minimum on the first day (0.000085) demonstrating an inverse
relationship with species diversity (Figure 4).
Similarly,
the phytoplankton densities oscillated over time. The maximum density occurred
at day 1, and then declined to an initial low of 12700 x103 indiv./L at day 6. Thereafter, densities oscillated widely
but with a somewhat declining consistently to a minimum of 9303 x103
individuals/L at day 15 (Figure 5).
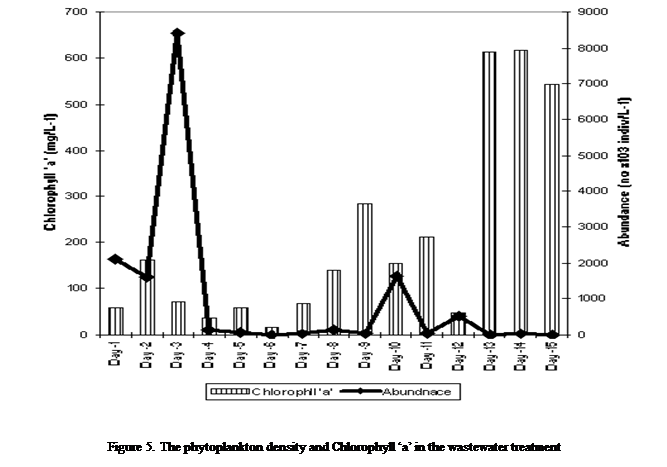
The
biomass values for chlorophyll ‘a’ fluctuated widely (irregularly) from day
1(59.51mg/m3) to day 12(47.75mg/m3).
On day 13, the biomass values increased sharply (613.29mg/m3) and
then declined on day 15 (542.33mg/m3) such that the chlorophyll ‘a’
levels in the wastewater increased greatly from day 1 to the end with
percentage increase from 15 - 89.0% (Figure 5).
The
different water quality attributes and phytoplankton descriptors during the
exposure period (t), were compared and the trend demonstrated series of
relationship such as the high positive correlation between pH and species
diversity (r2 = 0.59), and chlorophyll ’a’ (0.69), Dominant index and TDS (r2 = 0.63), Dominant index
and conductivity (r2 = 0.54), Dominant index and nitrate (r2 = 0.62), Dominant index
and abundance (r2 = 0.62), nitrate and log transformed (log x+1) phytoplankton
abundance (r2 = 0.85). Other positive relationships were observed between
dissolved oxygen and species diversity (r2 = 0.59) and chlorophyll
‘a’ (r2 = 0.69), temperature and dominant index (r2 =
0.50), and species diversity and chlorophyll a (r2 = 0.59) (Figure
6).
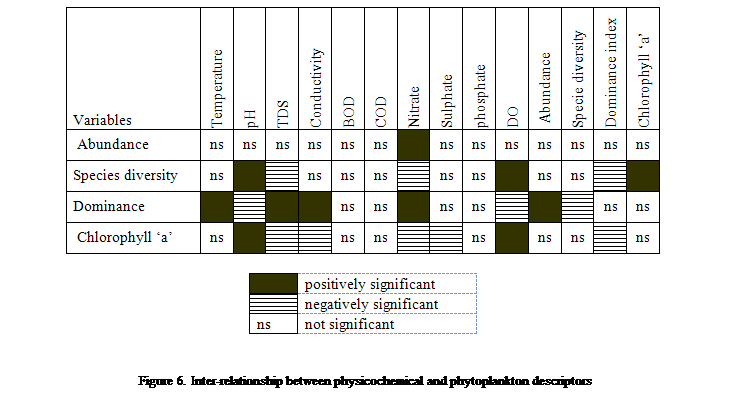
Negative
relationships also emerged in the pairing of attributes such as the
relationship between chlorophyll and conductivity (r2 = -0.72), TDS
(r2 = -087), phosphate (r2 = -0.80), nitrate (r2
= -0.57), SO4-2 (r2 = -0.62), dominance (r2
= -0.67), dominance and species diversity (r2 = -0.98), pH(r2
= -0.68), DO(r2 = -0.64); and species richness and TDS (r2
= -0.54), and nitrate (r2 = -0.63) (Figure 6).
In the suite of phytoplankton
descriptors, regression dominant index (r2 = 0.53) had the most
significant relationships between the actual and the predicted, followed by
chlorophyll a’ (r2 = 0.48, species diversity (r2 = 0.47),
and phytoplankton abundance (r2 =0.38) (Figure 7 (a-d)).
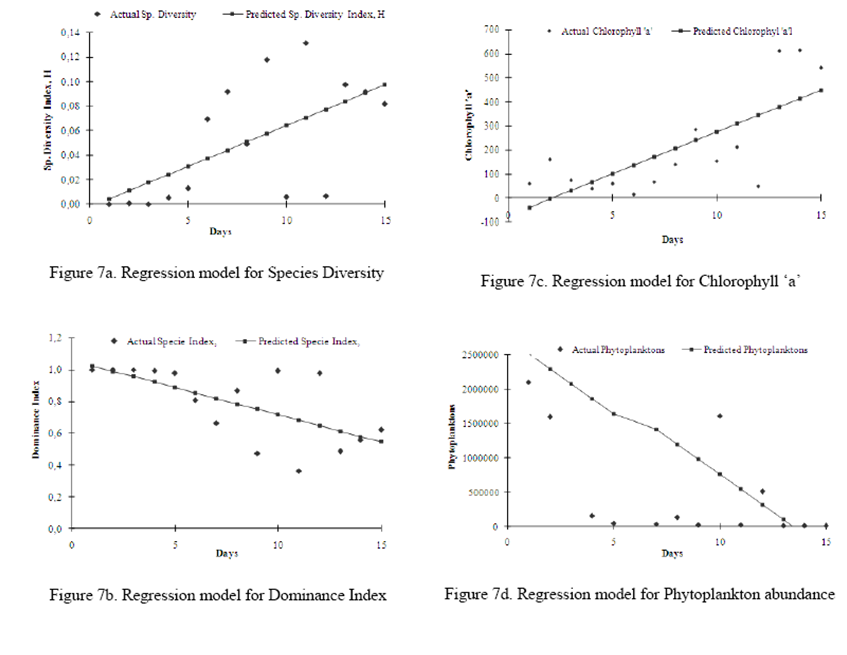
Also, an attempt was made to
find out the model that would adequately correlate the experimental data that
could be used for easy prediction and efficient future studies in the field.
The regression model was used to predict the responses between dependent and
independent variables, which gave rise to the following relationships such as
the relationship between species diversity and chlorophyll ‘a’ being represented
as species diversity = 0.024 + 0.00013 (chlorophyll ‘a’), where r2 =
0.320, n = 15 (Figure 8a). Also, dominance index and chlorophyll ‘a’ is
represented as dominance index = 0.9360 – 0.000734 (chlorophyll a), where r2
= 0.4458, n = 15 (Figure 8b).
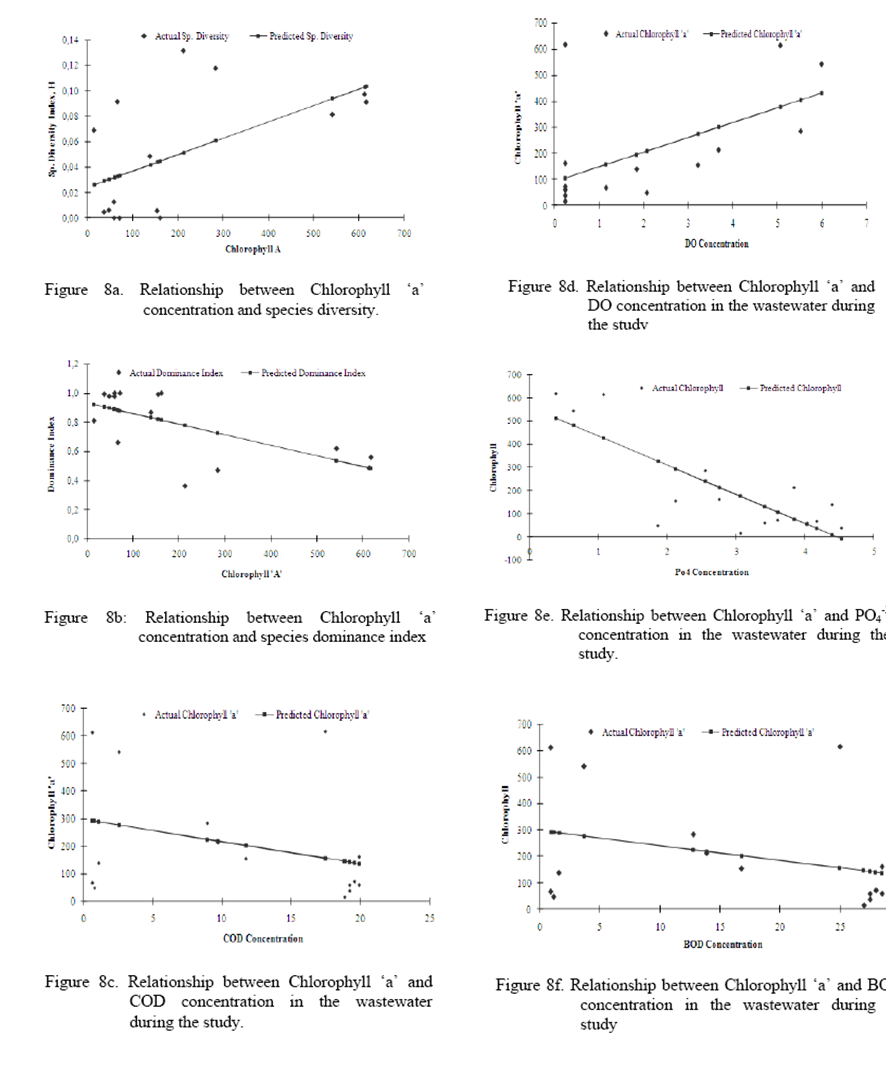
The
relationship between chlorophyll ‘a’ and COD as Chlorophyll a = 297.74 – 8.12
(COD), where r2 = 0.10, n = 15 (Figure 8c). Chlorophyll ‘a’ and DO
is represented as Chlorophyll ‘a’ = 91.21 + 56.76, (DO) where r2 =
0.32, n = 15 (Figure 8d).
Chlorophyll
‘a’ and BOD5 is represented as Chlorophyll ‘a’ = 297.77 – 5.68 (BOD5),
where r2 = 0.10, n = 15 (Figure 8e).
Chlorophyll ‘a’ and PO4-3 is represented as
Chlorophyll ‘a’ = 561.86 – 125.696 (PO4-3), where r2
= 0.649, n = 15 (Figure
In
addition, the multiple linear regression between variables such as chlorophyll
‘a’, BOD5, DO, and pH is defined by the linear equation: Chlorophyll
a = -2201 + 6.49 (BOD5) + 41.28 (DO) + 280.28 (pH), where r2
= 0.5815, n = 15.
From
the above equation the chlorophyll a concentration in the wastewater increases
by the factors 6.49 per unit BOD5 in the water, 41.28 per unit
increase in DO and 280.28 per unit increase in pH.
These variables (BOD5, DO and pH) contribute to chlorophyll a
variation in the wastewater. However, only 58% of the changes in chlorophyll a
values can be attributed to the BOD5, DO and pH values based on the
coefficient of determination, r2.
Similarly, the relationship
between chlorophyll ‘a’, and other biological variables (dominance index,
species diversity and abundance) in the wastewater is defined by Chlorophyll
‘a’ = 2592.05 - 2429.66 (dominance index) - 9225.48 (species diversity) -
0.000012 (abundance), where r2 = 0.6255, n = 15. The level of
chlorophyll ‘a’ in the wastewater decreased by the factors - 2429.66, -9225.48
and - 0.000012 per unit decrease in dominance index, species diversity and
abundance respectively in the wastewater. Only 62.7% of changes in chlorophyll
‘a’ can be attributed to dominance index, species diversity and abundance of
phytoplankton community based on the coefficient of determination r2.
However, the chlorophyll a concentration might be partly attributed to
dominance index, species diversity and abundance.
Finally,
the relationship between chlorophyll ‘a’ and nutrient related variables (NO3-,
PO43- and SO42-) is defined by the
linear equation:
Chlorophyll
‘a’ = 608.8 – 344.53 (NO3-) - 104.78 (PO43-
- 2.187(SO42-), where r2 = 0.714, n = 15. The
chlorophyll ‘a’ concentration in the wastewater increased by the factors -
344.53, - 104.78, and 2.187 per unit decreases in NO3-, PO43-and
SO42- respectively. The chlorophyll a concentration is
partly dependent on the NO3-, PO43-and
SO42- concentrations in the wastewater. About 71.4% of
the changes in chlorophyll a can be attributed to NO3-,
PO43- and SO42- based on the
coefficient of determination r2.
DISCUSSION
Recently,
research efforts by ecologists are geared towards preventing and solving
environmental problems especially those related to human interference such as
domestic and industrial wastes that are discharged into the natural
environment. Part of these processes is in the domain of ecological management
and restoration, predominantly on waste management, to restore habitat
integrity through sustainable restoration principles and practice procedures.
Understanding the intricate progression of the physicochemical and biological
processes in a wastes treatment system is imperative to more efficient
management processes. In this respect, this study evaluated biological
development in a natural treatment system utilizing solar radiation as energy
source. (Berna et al., 1986)
Thus the early stages of this
study demonstrated low phytoplankton species richness and high abundance
against higher species richness and relatively depressed abundance at the later
stages that is posited to be associated with the preponderance of few blue
green species in the population that have competitive ability to out
competed and exterminate the other less
tolerant species in the community on account of its ability to produce
extracellular substances that are capable of inhibiting the survival and development
of other phytoplankton species. Thus the less resistant species that were
unable to withstand the stress or unfavourable
conditions were eliminated (Chindah, 1998). This
implies that the few but dominant species (Merismopedia punctata, Anacystis
aeuroginosa, Romeria elegans) found in the population at the early stages
were species that have competitive ability and/or resilience taxa, competent of
producing extracellular substance that inhibited and or eliminated other algal
species. These species that can be referred as resistant species, could serve
as bio- indicators for municipal waste for the eco-region (Fogg,
1962). Javanmardian
and Palsson (1992) had reported similar inhibitory
effects of some of these blue green algal species at high density from
municipal wastewater. Darley (1982) posited that such inhibitory effect may as
well be attributed to reduction in photosynthetic efficiency, self shading and accumulation of auto-inhibitors, since
transparency is usually affected by the phytoplankton density and nonliving
suspended matter. This contention may result in the
reduction of the amount of light impinging and reaching the phytoplankton for
photosynthesis (Abdel-Aziz et al.,
2001). The scenario may be responsible for the few species richness observed at
the early stages when the blue green algal density was high in the
treatment waste water (Dorgham et al., 2004).
Analogous
to this, is the successional pattern observed with the attendant progression in
the recruitment of species as the condition of water quality improved
especially as nutrient load declined. The sequence of entrants of species into
the population by new colonist and or the reemergence of species inadvertently
is responsible for the increased species richness and dominance of particular
individuals which is similar to the observation reported on nutrient enrich
system comparable to wastewater (Hillebrand and Sommer, 1997, 2000; Vymazal, 1988). This circumstances displayed in the emergence
of species is an indication of the species preference for a particular water
quality to thrive.
These attributes may be answerable to the changes observed in the
community structure (from a single Cyanophyceae community at the start of the experiment to
a more complex Bacillariophyceae, Chlorophyceae
and Euglenophyceae at the termination of the study) is another substantiation that the Cyanophyceae species observed were opportunistic in
nature (Chindah, 1998). It is therefore palpable that
the occurrence and ascendancy of Bacillariophyceae
and the emergence of other taxonomic groups in the phytoplankton community is
adjudged as a clear indication of the recovery status of the water quality (Chindah et al.,
2005).
In contrast to the results of similar studies on waste water in Europe
-Spain where Chlamydomonas sp. was the
dominant taxa (Soler et al., 1991), Anacystis aeuroginosa was observed as the dominant species. The
variability in dominant species in the wastewater type may be associated with
the nature and characteristics of the waste water as it appeared that the
effluent quality visibly influenced the kind of phytoplankton species. Some of
these phytoplankton species observed in this study have been implicated in
organic waste polluted environment (Amadi et al., 1997). Interestingly, these sequences of events seem to have remarkable effect on the species diversity and
dominant index of the phytoplankton species as increase in diversity was
observed at the early successional stages but the arrival of new colonist and
perhaps competition accounted for the decreased diversity at the later
successional stage.
These
marked changes observed in this study point to the important role of
competitive displacement on temporal species assemblage and occupancy in the
wastewater system with adjustment in the physicochemical quality status or as
recovery period progresses and to a large extent explains the critical
requirement of phytoplankton environment as it tended to improve as equilibrium
in the water parameters is achieved.
This is attributed to the
reduction of the inhibitory substances which was not determined in this study
and improvement of the water quality attributes (Vymazal, 1988; Javanmardian and Palsson, 1992)
and perhaps the influence or effect of other parameters such as temperature,
solar energy and increased oxygen concentration (Berna
et al., 1986).
The
critical associations observed between the independent and dependable variables
highlight the importance of water quality and environmental gradient on the
organization of biological resources and the close relationship between the
predicted and actual data implies that these parameters can be relied upon in
waste water treatment monitoring as it provide understanding of the possible
ecologic effects of anthropogenic activities and ecosystem stability. It is the believe
of the authors that the study provided a
framework in which ecological processes can be manipulated to achieve a desired
phytoplankton community that identifies successional activities and dynamic factors
influencing succession in a restoring singularly applied treatments
ACKNOWLEDGEMENTS
We wish to thank the staff of
the Institute of Pollution Studies (IPS) Rivers State University of Science and
Technology, Port Harcourt especially U. J. Ikoro,
Hanson Uyi, Nathan Nario
and Uchenna Anireh for
their support and assistance during the laboratory studies. The authors also
acknowledge with thanks the constructive, thorough, and valuable comments by
eight anonymous reviewers of this manuscript.
LITERATURE CITED
Abdel-Aziz N. E.; M. A. Fahmy and M. M. Dorgham. 2001, Hydrography,
nutrients and plankton abundance in the hot spot of Abu Qir Bay, Alexandria,
Egypt, Medit. Mar. Sci. 2 (2): 17-31.
Ajayi, S. O. and O. Osibanjo. 1981. Pollution Studies on Nigerian Rivers. II.
Water Quality of some Nigerian Rivers. Environ. Pollut.
2: 87-95.
Amadi E. N.; A. C. Chindah and C. C. Ugochi. 1997.
The effect of municipal drainage on the microflora of
a black water stream in Port Harcourt, Nigeria. Niger Delta Biologia
2 (1): 125-139.
American Public Health Association (APHA). 1998. Standard methods for
the evaluation of water and waste water. 2th ed,
Washington , D. C. Americam Public Health.
Association.
Berna, L. M.; M. Llorens, F. Torrella Mateu, I. Martínez y A. Soler. 1986. Estudio
de la autodepuración microbiológica y físico-química de aguas residuales por lagunaje profundo. Anales de Biología 10: 49-60.
Chellapa N. T. 1990. Phytoplankton
species composition, chlorophyll biomass, and primary production of the jundai reservoir (north eastern Brazil) before and after entrophication. J. Acta. Hydrobiol. 32:
75-91.
Chindah, A. C. 1998. The effects of
industrial activities on the periphyton community at
the upper reaches of New Calabar River, Niger Delta.
Nigeria. Water Resources 32 (4): 1137-1143.
Chindah, A. C. and J. Pudo.
Chindah, A. C. and O. C. Sibeudu. 2003. Levels of hydrocarbons and heavy metals in
sediment and a decapod crustacean (Crab - Uca
Tangeri ) in the Bonny/New Calabar
River Estuary, Niger Delta. Pol. Journal of Environmental Protection 25/26:
55-71.
Chindah, A. C.; S. A. Braide and E. Izundu. 2005.
Treatment of Municipal wastewater quality using sunlight. Caderno de Pesquisa 17 (2):
27-45.
Darley, W. M. 1982. Algal Biology. A Physiological Approach. Basic
Microbiology, 9, 168. Blackwell
Scientific Publications, Oxford, UK.
Dorgham, M. M.; N. E. Abdel Aziz, K. Z. El-Deeband M. A. Okbah. 2004
Eutrophication problems in the Western Harbour of
Alexandria, Egypt Oceanologia 46 (1): 25-44.
Durand, J. R. and C. Leveque. 1980. Flore et Faunae Aquatiequies de l’
Afrique. Cah Off Res. Sci. Tech.Outre
Mor. 1: 5-46.
Ekweozor, I. K. E.; A. Ugbome and E. I. Ombu. 1987. The
effects of chronic oil pollution in the central Bonny esturary.
Proceedings of 1987 seminar on the Petroleum Industry and the Nigerian Environmnet, Nov. 9 – 12 PortHarcourt.
198-207.
Fogg G. E. 1962. The importance of
extracellular products of Algae in the aquatic environment. In biological
problems in water pollution. Third seminar, August 13-17,1962 pp.34-37.
(ed. Clarence .M. Tarzwell).
US Dept. of Health education and Welfare Public Health Service Water Supply and
Pollution control Cincinnati, Ohio USA.
Gobo, A. E. 1988. Relationship between rainfall trends and flooding in
the Niger Delta Benue basin. J. Metrology 13 (37): 813-828.
Hillebrand, H. and U. Sommer. 1997. Response of epilithic
microphytobenthos of the Western Baltic sea to insitu experiments with nutrient enrichment. Mar. Ecol. Prog. Ser. 160: 35-46.
Hillebrand, H. and U. Sommer. 2000- Diversity of benthic microgalae
in response to colonization time and eutrophication. Aquatic Botany 67:
221-236.
Ibiebele D. D.; S. A. Braide, A. C. Chindah and F. O.
Harry. 1987 . Oshika oil spill incident: Case study
four years after the spill. In
Proceedings of the 1987 Seminar on the Petroleum Industry and the Nigerian. Environment.
pp. 126-132.
Institute of Pollution Studies (IPS). 1990. Ecological post impact
studies of Ebubu-Ochani oil spillage. Institute of
Pollution studies Rivers State University of Science and Technology, Port
Harcourt Rivers State of Nigeria. RSUST/IPS/TR/90/02, 232 pp.
Javanmardian, M. and B. O. Palsson. 1992. Continuous photoautotrophic cultures of the
eukaryotic alga Chlorella vulgaris
can exhibit stable oscillatory dynamics. Biotechnol. Bioeng, 39: 489-497.
Joiris C, and M. I. Azokwu. 1999. Heavy metals in the bivalve Anadara (Senilia senilis) from Nigeria. Mar. Pollut.
Bull. 38 (7): 618-622.
Margalef, R. 1958. Information theory
in ecology. Gen. Sys. 3: 50-71.
Mills F. W. 1932. Some diatoms from Warri River Southern Nigeria. J
Royal Microsc. 853: 382-395.
Ndiokwere, C. L. 1984. An investigation
of heavy metal content of sediments and algae from the River Niger and Atlantic
coastal water. Environ. Pollut. (B) 7: 247-254.
Ogan, M. T. 1988. Examination of
surface water used as source of supply in PortHarcourt
area: II. Chemical hydrology. Arch. Hydrobiol. 79
(2/3): 25-342.
Ogamba, E. N. 2003. Water quality
status of Elechi Creek complex in relation to
physicochemical parameters and plankton distribution. Ph.D
Thesis, Department of Applied and Environmental Biology, Rivers State
University of Science and Technology, Port Harcourt, Nigeria
Okpokwasili, G. C. and C. N. Nwabuzor. 1988. Primary biodegradation of anionic
surfactants in laundry detergents. Chemosphere 17: 2175-2182.
Okpokwasili, G. C. and A. C. Olisa. 1991. Riverwater biogradation of surfactants in detergents and shampoos.
Water Res. 25 (11): 1425-1429.
Partrick, R. and C. Reimer. 1966. The
diatoms of the Uninted States exclusive Alaska and
Hawaii, Eunotiaceae, Achnanthaceae,
Naviculaceae. Philadelphia. Livingstone Publ. Com.
Powell, C. B. 1987. Effects of freshwater oil spillages on fish and
fisheries. The Proceedings of 1987 seminar on the petroleum industry and the
Nigerian environment.
Sieminska, J. 1964. Chrysophyta
II. Bacillariophyceae Okrzemkii:
In: Starmach
K (ed.) Flora S lodkuwodn: Polski
(Freshwaterr Flora of Pokind)
6. Warszawa, Pan'stwowe Wydawinctwo
Naukowe. 610 pp.
Soler A.: J. Saez, M. Llovens, I. Martinez, F. Torrella
and L. M. Berna. 1991. Changes in physico-chemical
parameters and photosynthetic microorganisms in a deep wastewater
self-depuration lagoon. Wat. Res.25 (6): 689-695.
Starmach, K. 1966. Cyanophyta‑Since,
Glaucophyta‑ glaukifity
In: Flora. Slokuwodna
Polski. Vol. 2 (ed. Starmarch,
K.). Pan'stwowc Wydawinctwo
Naukowe, Warzawa. 808 pp.
Vymazal, J. 1988. The use of periphyton communities for nutrient removal from polluted
streams. Hydrobiologia 166: 225-237.
Página diseñada por Prof. Jesús Rafael Méndez Natera
TABLA DE CONTENIDO DE LA REVISTA CIENTÍFICA UDO
AGRÍCOLA



