Revista Científica UDO Agrícola Volumen 7. Número 1. Año 2007. Páginas: 181-194
Effect of crude oil on the development of mangrove (Rhizophora mangle L.) seedlings from Niger Delta, Nigeria
Efecto del petróleo crudo sobre el desarrollo de
plántulas de mangle (Rhizophora
mangle L.) en el Delta de Niger, Nigeria
Alex
Chuks CHINDAH![]() 1, Solomon Amabaraye
BRAIDE1, Jonathan AMAKIRI2 and Judith ONOKURHEFE1
1, Solomon Amabaraye
BRAIDE1, Jonathan AMAKIRI2 and Judith ONOKURHEFE1
1Institute
of Pollution Studies. Rivers State
University of Science and Technology. Nkpolu Oroworukwo. P. M. B. 5080, Port Harcourt. Rivers State, Nigeria and 2Plant Science and
Biotechnology. University of Port Harcourt, Port
Harcourt, Nigeria.
E-mails: alexchindah@yahoo.com and
alexchindah@hotmail.co.uk ![]() Corresponding author
Corresponding author
|
Received: 09/04/2007 |
First reviewing ending: 10/24/2007 |
First review received: 11/10/2007 |
|
Second reviewing ending: 11/21/2007 |
Second review received: 12/11/2007 |
Accepted: 12/13/2007 |
ABSTRACT
This
study was designed using randomized
block design to evaluate the acute and chronic effects of crude oil (Bonny
Light) on the growth performance of mangrove seedlings in a 16-week laboratory
experiment monitoring critical plant growth attributes such as stem height and diameter, leaf length
, width and numbers of leaves (leaf production), senescence and seedlings survival. Two
treatments were compared with the control (no oil added); they were: 150 mL
crude oil applied once and 15 mL crude oil applied weekly. The results showed
differences in response of seedling attributes exposed to the different
treatments with acute exposure having a
declining response pattern of stem height > stem diameter > leaf length =
leaf and chronic exposure with leaf length > stem height > leaf width
> stem diameter. These results were further corroborated by cluster and correspondence
analyses, and demonstrated affinities of the attributes and extent and
sensitivity of each attributes. This
suggests that the mangrove seedlings respond differently to various crude oil
exposures which has implications for
restoration activities. The present study demonstrated that mangrove seedlings
are negatively impacted by both acute and chronic exposure but more so with
seedlings under acute exposure and further provided insight on the potential ecological
risk associated with mangrove seedling development exposed to crude oil
contamination.
Key words: Rhizophora mangle,
mangrove seedling, Bonny light crude oil, Niger Delta, Toxicity
RESUMEN
Este estudio se diseñó para evaluar
los efectos agudos y crónicos del petróleo (Bonny Light)
sobre el comportamiento del desarrollo de plántulas de mangle bajo condiciones
de laboratorio, monitoreando las características críticas del crecimiento de
las plantas tales como altura y diámetro del tallo, longitud de hojas, ancho y
número de hojas (producción foliar), senescencia y sobrevivencia de plántulas
durante 16 semanas. Los resultados mostraron diferencias en la respuesta de las
características de las plántulas expuestas a los diferentes tratamientos con el
efecto agudo teniendo un patrón de respuesta descendente de la altura del tallo
- tasa de crecimiento relativo (TCR) = 0,17 > diámetro del tallo -
TCR = 0,01 > longitud de hojas – TCR = 0 = ancho de hojas – TCR = 0 y el
efecto crónico con longitud de hojas - TCR = 0,20 > altura de tallo – TCR =
0,19 > ancho de hojas – TCR = 0,15 > diámetro del tallo – TCR = -0.03.
Estos resultados fueron adicionalmente corroborados mediante análisis de
agrupamiento y correspondencia. Los resultados sugieren que las plántulas de
mangle respondieron diferentemente a las varias exposiciones de petróleo y
suministraron evidencia del comportamiento de las plántulas, la supervivencia y
la implicación de las actividades de restauración a varios niveles de
exposición de petróleo.
Palabras
claves: Rhizophora mangle,
plántulas de mangle, petróleo crudo, Delta del Niger,
Nigeria
INTRODUCTION
Nigeria has the third largest mangrove forest in the world
and the largest in Africa (9,730 km2) occupying and the lower stretches of the
southern limit of the Niger Delta and covering between 5,400 km2 and
6,000 km2 (NDES, 2000). There are three main mangrove families (Rhizophoraceae, Avicenniaceae
and Combretaceae) comprising
six species, namely: Rhizophora
racemosa G. Mey, Rhizophora mangle L., Rhizophora
harrisonii Leechem.,
Languncularia racemosa Gaertn, Avicennia germinans L. and
Conocarpus erectus L., and
the exotic family Palmae
(Arecaceae)
that is rapidly spreading across the Niger Delta (RPI, 1985; NDES, 1996 and
2000; NDDC, 2004). Another important component of the mangrove vegetation is
the exotic Nypa palm (Nypa fruticans Wurmb)
of the family Palmae introduced from Singapore
Botanical Gardens to Calabar in 1906 and Oron in 1912 (Keay el al., 1964). The red mangrove
constitutes over 60% of the mangrove area cover in the region.
The
mangrove plants (Rhizophora mangle L.) are salt tolerant species
that grow on sheltered shores in the
tropics and sub-tropical estuaries (IPIECA, 1993), where they
provide ecosystem functions and several human utility benefits especially for
coastal communities of Niger Delta (Nigeria). Their halophytic nature and ability to compensate for low oxygen in the
soil allows them to flourish in the environment (Choudhry,
1997). However, their complex breathing roots make them vulnerable to crude oil
that can block the openings of the breathing roots. This has
posed serious threats to mangrove plants.
Crude oil plays an important role in the economy of Nigeria
and about 70% of oil exploration and exploitation activities take place in the
mangrove areas of the Niger Delta. However, mangrove forest clearing and oil
spills from operational failures and vandalism of pipelines, oil well blowouts,
tanker seepages and accidents and deblasting
operations contribute to mangrove species loss and degradation of the ecosystem
(Imevbore, 1979 and 1981; Baker, 1981a,b; Ekweozor 1985 and 1989,
Snowden and Ekweozor, 1987; Nnyong
and Antia, 1987, Amadi et
al., 1996).
The crude oil spilled into the mangrove environment through
tidal influences that characterize the ecosystem provides for wider dispersal
and distribution in the intertidal flat areas resulting in the deposition of
crude oil on the aerial roots and sediment (Baker, 1981a). Thus,
crude oil covers the breathing roots and pores, thereby asphyxiating the
sub-surface roots that depend on the pores for oxygen transfer (Odu et al.,
1985). This in turn impairs the normal salt exclusion process resulting in
accumulation of excess salt in the plant contributing to enhancing the stress
condition of the plant and ultimately, to death, loss of mangrove plants,
habitat destruction and degradation (Imevbore, 1979).
Of the four main ecological areas in the Niger Delta
(mangrove, freshwater swamp forest, lowland and barrier island swamp forest)
the mangrove is the most affected by oil exploration and exploitation as it has
very poor regeneration potential. This scenario generates concern among the
different stakeholders on the need of revegetating the degraded mangrove habitat
On account of this, mangrove plants are vulnerable and
undergo steady unpalatable declining
quality and functions in the integrity of the ecosystem. The continuous oil
activity in the region and accidental crude oil spills into the mangrove
ecosystem are the genesis of the scientific motivation to examine the acute and
chronic effects of Bonny light crude oil on the development of mangrove
seedlings of Rhizophora mangle using
growth attributes (such as stem growth, seedlings survival, leaf production and
senescence, as surrogates ).
MATERIALS AND METHODS
Description of
Study Area
The study was conducted at Eagle Island located at the
upper reach of Bonny estuary of the eastern Niger Delta, Nigeria and lies
within longitude 4º
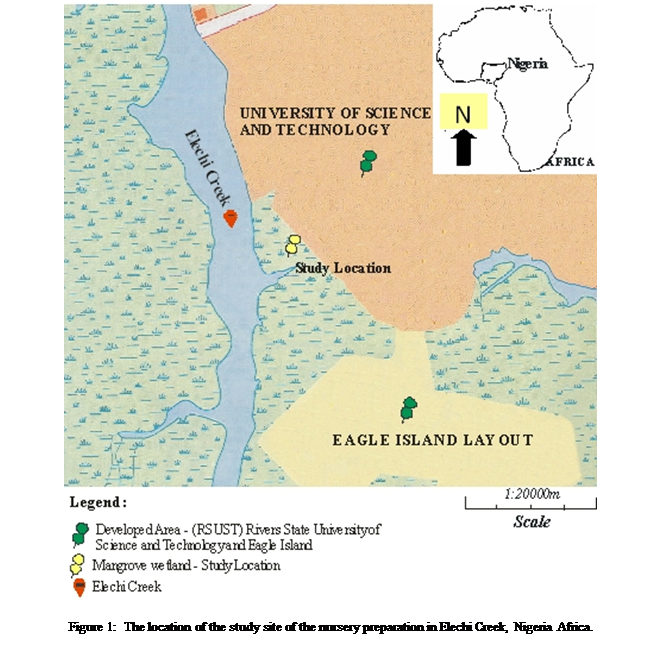
Vegetation in the area was characteristically mangrove,
with the dominant types being red mangrove (Rhizophora
racemosa), white mangrove (Avicennia
africana) and black mangrove (Laguncularia
racemosa). The area was also inhabited by other
plants (e.g fern -Achrostichum
aureum and grass-Paspalum
varginatum) and animals (e.g. mud skipper Periophthalmus sp., fidder crabs Uca tangeri
and Periwinkles).
The climate of the area was basically that of equatorial
tropical rainfall occurring throughout most of the year except for the months
of December, January and February which comprised the dry season. The annual
rainfall in the area was about
Economic
activities by human in this area were mainly, fishing, trading and transportation.
The sampling sites (10 x
Treatments
Treatment
was by applying the Bonny light crude oil (BLC) that commenced at the end of 60-day acclimation period. The
crude oil (Bonny Light Crude -BLC) constitutes of n-alkane-containing
oil such as saturates (56%), aromatics (31%), polars (11%), and asphaltenes
(2%), it also has 35.3° API gravity and contains 0.1% sulphur
content (Norman et al., 2004).
The acute treatment, consisted of a one-time application of
120
mL crude oil (Bonny Light crude oil) added on the surface of the mud. The
chronic treatment consisted of weekly application of smaller amount (15ml) of
the same crude oil (Monaghan and Koons, 1975 and Proffitt et al.,
1995).
Stem height, stem girth (diameter) at the first inter-node, number of nodes, number of leaves, and leaf area (length, and width), were measured individually using vernier calipers; the fate and growth of seedlings were monitored weekly for 16 weeks. Any yellowing of leaves and seedling survival were recorded. The response patterns of mangrove seedlings among treatments were examined by hierarchical cluster analysis on log (x + 1) transformed data using JMP IN analytical software (Clarke and Gorley, 2001, 2006). Group average sorting (= unweighted pair-group method; (Sneath and Sokal, 1973) was used as the clustering method and Bray–Curtis similarity for resemblance measure (Bray and Curtis, 1957). Results were expressed as a dendrogram in which samples were ordered into groups.
RESULTS
Seedling Survival
Acute
treated plants demonstrated seedling mortality on 2nd and 3rd
week corresponding to 90% and 80% survival, respectively. No further mortality
occurred until the 9th week when 30% loss was observed culminating
in 70% survival (Table 1, Figure 2a). The relationship within the acute treated
seedlings was not significant (r = 0.02), as well as the difference between
treated and control seedlings (Wilcoxon sign rank Z = 60.3 > P = 0.21(0.05)).
Seedlings under chronic treatment did not show any mortality such that 100%
survival was observed at the end of the experiment (16 weeks). Similarly, 100%
survival pattern was observed for the control seedlings (Table 1, Figure 2a).
|
Table 1. Linear regression equations for
relationships for each treatment on mangrove (Rhizophora mangle L.) growth
characteristics of seedlings exposed to different crude oil (Bonny Light) treatments (acute, chronic
and control) in the Niger Delta, Nigeria. |
|||
|
Plant Attributes |
|
Relationship |
R2 |
|
Leaf production |
Control |
y = 0.1105x + 6.08 |
R2 = 0.58 |
|
Acute |
y = 0.2819x + 4.82 |
R2 = 0.89 |
|
|
Chronic |
y = 0.1105x + 6.08 |
R2 = 0.58 |
|
|
|
|||
|
Seedling
survival |
Control |
y = 10 |
R2 =0.00 |
|
Acute |
y = -0.174x + 9.45 |
R2 = 0.78 |
|
|
Chronic |
y = 10 |
R2 = 0.00 |
|
|
|
|||
|
Stem height |
Control |
y = 0.9429x + 51.26 |
R2 = 0.98 |
|
Acute |
y = 0.8814x + 116.66 |
R2 = 0.97 |
|
|
Chronic |
y = 1.3306x + 69.21 |
R2 = 0.99 |
|
|
|
|||
|
Stem diameter (girth) |
Control |
y = 2.2377x - 9.43 |
R2 = 0.75 |
|
Acute |
y = 2.174x + 1.08 |
R2 = 0.95 |
|
|
|
Chronic |
y = 2.4191x - 5.13 |
R2 = 0.95 |
|
|
|||
|
Leaf length |
Control |
y = 1.6441x + 45.06 |
R2 = 0.97 |
|
Acute |
y = -0.2292x + 54.10 |
R2 = 0.06 |
|
|
Chronic |
y = 1.6349x + 42.58 |
R2 = 0.97 |
|
|
|
|||
|
Leaf width |
Control |
y = 0.7968x + 16.14 |
R2 = 0.97 |
|
Acute |
y = -0.0572x + 20.07 |
R2 = 0.04 |
|
|
Chronic |
y = 0.6734x + 13.91 |
R2 = 0.98 |
|
|
|
|||
|
Senescence |
Control |
y = 2.4191x - 5.125 |
R2 = 0.95 |
|
Acute |
y = 2.174x + 1.08 |
R2 = 0.95 |
|
|
Chronic |
y = 2.4191x - 5.125 |
R2 = 0.95 |
|
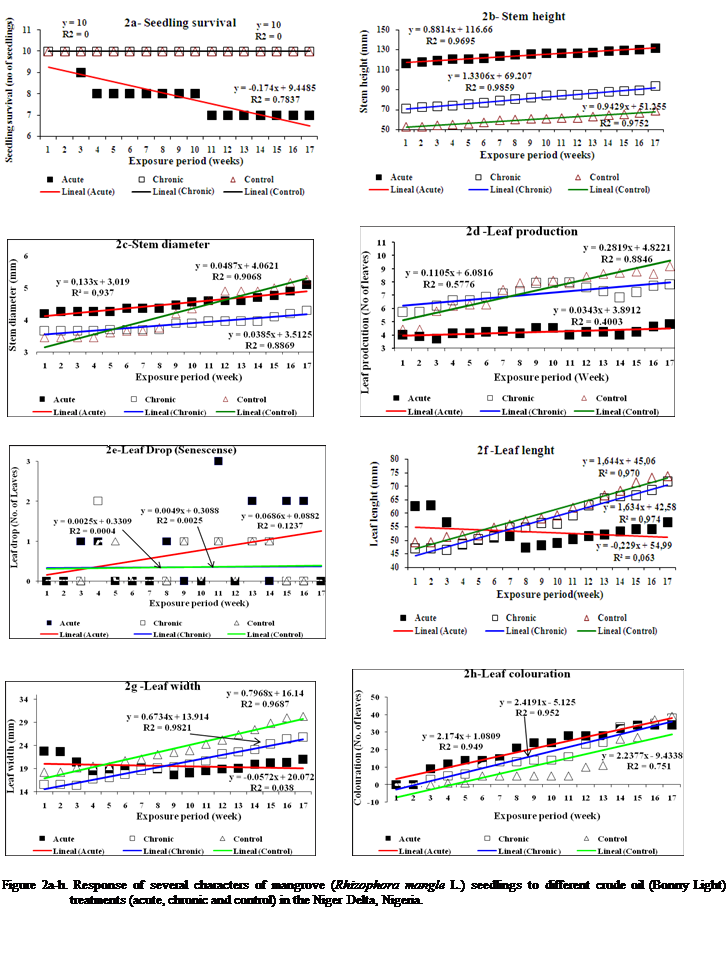
Stem Growth (Height)
R. mangle seedlings exposed to acute
crude oil treatment exhibited
a 7.93% increase in height during the first nine weeks of the study; but showed
little growth thereafter. Stem growth (height) increased slowly, but steadily
in a near-linear fashion (Table 1, Figure 2b), achieving a total increase of
11.76% after 16 weeks.
While seedlings exposed
to chronic crude oil treatment demonstrated increases in stem growth (height)
from start (
Stem Girth (Diameter)
Stem girth for
acute exposed seedlings increased slightly from start (
For the chronic exposed
seedlings there was no observable increase in girth size until the 4th
weeks (
There was no observable change in girth from start to the 3rd
week (
Statistical assessments between
treated seedling and control demonstrated great similarity (acute r2
= 0.94; chronic r2 = 0.95, Table 1, Figure 2) and differences between treated and
control seedlings were not statistically significant (Acute - Wilcoxon
Sign-Rank z = < P(0.05)
; chronic Wilcoxon Sign-Rank z =
42.0 > P = 0.043(0.05)).
Leaves
Leaf Production (Number of Leaves)
Leaf production for the acute crude oil treatment on R. mangle demonstrated an unsteady
pattern, but an increase in the number of leaves was observed starting from
week 1 (40) to the end (week 16) of the experiment (48). Thirty-eight percent
of Rhizophora
seedlings produced new leaves while 62% did not record leaf production. Leaf
development (sprouting) started in the 3rd week; the maximum production was not
until the 3rd and 8th week (Table 1, Figure 2d).
Leaf
production for chronic crude oil treatment on Rhizophora mangle showed an unsteady pattern, with steady increase in the
number of leaves from week one (57) to the 10th week (80),
thereafter a decline to 78 at the end of the experiment with thirty two
percentages of treated seedlings producing new leaves and sixty eight (68%)
percentages did not record leaf production. Peak production was in the 8th
week (Table 1, Figure 2d).
However,
the control had consistent leaf production increasing from week one (44) to the
end of the experiment (92), more leaves was produced by the control seedlings.
Twenty eight percentages (28%) of Rhizophora seedlings produced new leaves while 72% did not
record leaf production. Leaf development (sprouting) started from the second
week and peak production was observed on the 3rd week (Table 1,
Figure 2d).
Leaf drop (Senescence)
Leaf
drop for acute treated mangrove plant (seedlings) was between (9 and 34 leaves)
with senescence commencing at the early stages of the experiment (week 2). The number
of shading increased almost exponentially to the end of the experiment (16th
week) and maximum shading of leaves was observed on the 14th week (Table 1, Figure 2e).
Leaf
drop for chronic treated seedlings lies between (3 and 38), while the control values
ranged from (1- 39). For the treatment, seedlings started shading leaves from
week three. The number shaded increased at intervals of six almost the same
number of leaves was observed to be shaded between weeks 3 to 4 (5), weeks 5 to
7 (13), weeks 8 to 9 (14), weeks 11 to 12 (24), weeks 13 to 14 (33 and 34) and
finally weeks 15 to 16 (35 and 38). Maximum shading of leaf was observed at the
end of the experiment (Table 1, Figure 2e).
The
control demonstrated the same pattern. Shading increased at interval of five
almost the same number of leaves was observed to be shaded at each of the
intervals between weeks 3 to 4 (1), weeks 5 to 10 (5), week 11 to 12 (10 and
11), weeks 13 to 14 (26 and 27), and finally weeks
15 to 16 (37 and 39). Maximum shading was recorded on the 16th
(Table 1, Figure 2e). The correlation
coefficient were moderately high (r = 0.93) and not significant (Wilcoxon
Sign-Rank, z = 57 > P = 0.01(0.01)).
Leaf Length
Chronic exposure of the mangrove seedlings to the crude oil had no
significant effect on leaf length (Table 1, Figure
Changes in leave length in treatment plants fluctuated widely
during the study period. There were noticeable changes in the leave length from
start (week 0) to first week (
However,
the control plants demonstrated a rather steady growth pattern with increases
from start week 0 (
Leaf Width
Similar
R. mangle seedlings exposed to acute
treatment decline in leaf width as reported for leaf length from start week (
Changes
in leave width in the chronic treated plant started from the 1st
week (
Leaf Colouration
R. mangle seedlings exposed to acute
treatment demonstrated 10% yellowish colouration (chlorosis) which was commenced from the second week. Also,
seedlings exposed to chronic treatment had 19% yellowish colouration
(chlorosis), while the control had 5% yellowing of
leaves (Chlorosis). The yellow colouration
for chronic and control commenced from the third week to the end of the 16th
week (Figure 2h)
Relative growth rate (RGR)
The relative growth rate for
the seedling treatments indicated a better growth performance by the chronic
than the acute treatment with respect to the control (Table 2). The RGR
response value for acute treatment follow a pattern of stem height (0.17) >
stem diameter ( 0.01) > leaf length (0) =
leaf width (0) ,while chronic and control had similar RGR pattern of
leaf length (RGR = 0.20) > stem height (RGR = 0.19) > leaf width (RGR =
0.15) > stem diameter (RGR = -0.03) and leaf length (RGR = 0.20) > stem
height (RGR = 0.17) > leaf width (RGR = 0.16) > stem diameter (0.04)
respectively (Table 2).
|
Table
2. Relative growth rate of mangrove (Rhizophora mangle
L.) seedlings exposed to different crude
oil (Bonny Light) treatments
(acute, chronic and control) in the Niger Delta, Nigeria. |
|||
|
|
Treatment |
||
|
Parameter |
Acute |
Chronic |
Control |
|
Stem height |
0.17 |
0.20 |
0.17 |
|
Stem diameter |
0.01 |
-0.03 |
0.04 |
|
Leaf length |
0.00 |
0.20 |
0.20 |
|
Leaf width |
0.00 |
0.15 |
0.16 |
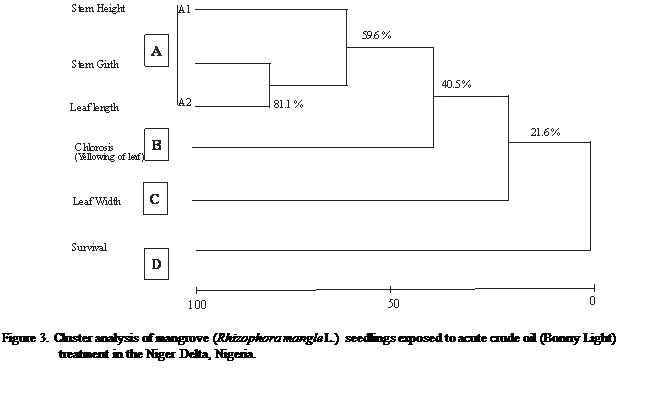
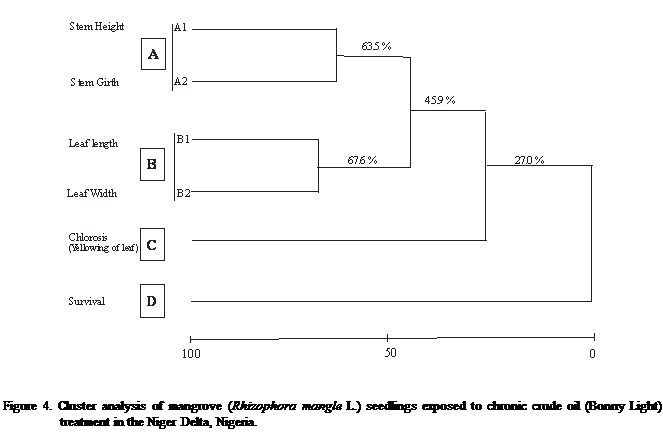
Similarity analysis carried
out with the use of the average method and Euclidean distance measure for acute
and chronic treatment examined responses of the plant attributes on the
different exposure. There was a relative divergent response of the attributes
on the mangrove seedlings which yielded four major results, denoted as A, B C
and D. For the acute treatment, the highest response was between stem girth and
leaf length (A-1, 81.1%) followed by stem height (A-2, 59.6%), yellowing of
leaf (B, 40.5%), leaf width (C 21.6%) and seedling survival (D, 0%) in that
decreasing response (Figure 3). While the chronic treatment indicated leaf
length and width (B, 67.6%) followed by stem height and stem girth (A, 63.5%),
yellowing of leaf (C, 27.0%) and seedling survival (0%) in that decreasing
order of response (Figure 4).
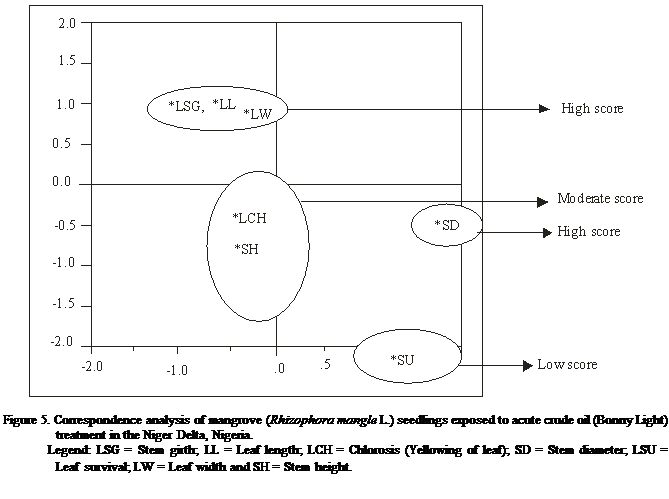
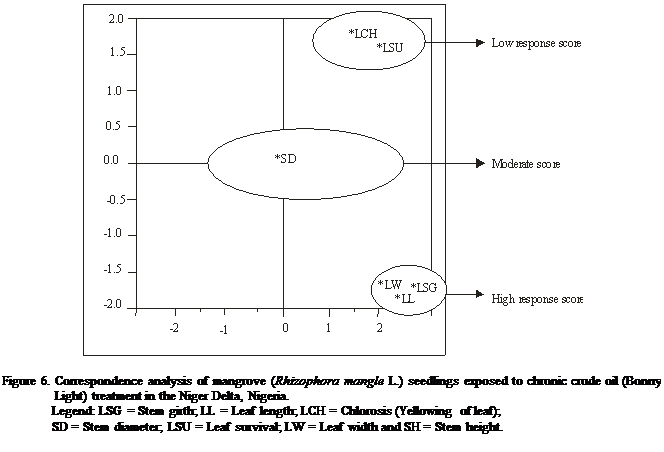
The correspondence analysis
corroborated the findings observed with the cluster analysis and reveal high
homogeneity between stem girths and leaf length that had high response score,
with stem height and yellowing of leaf having moderate response score while
seedling survival had very low response score for acute treatment (Table 3 and
Figure 5). Correspondingly, leaf length, leaf width and stem height and girth
for chronic treatment with relatively high response score, while yellowing of
leaf and seedling survival had low response score (Table 3 and Figure 6).
|
Table 3. Total structure coefficients of mangrove (Rhizophora mangle L.) seedlings exposed to acute and chronic crude oil (Bonny Light)
treatments in the Niger Delta, Nigeria |
||
|
Variable |
Acute |
|
|
C-1 |
C-2 |
|
|
Stem growth (height) |
-0.50 |
-0.91 |
|
Girth (diameter)
|
-1.35 |
0.98 |
|
Leaf length |
-0.58 |
1..01 |
|
Leaf width |
-0.25 |
0.94 |
|
Colouration |
-0.42 |
-0.18 |
|
Survival |
-1.18 |
-1.99 |
|
Variable |
Chronic |
|
|
C-1 |
C-2 |
|
|
Stem growth (height) |
2.22 |
-1.98 |
|
Girth (diameter)
|
-0.01 |
0.02 |
|
Leaf length |
2.48 |
-1.91 |
|
Leaf width |
2.03 |
-0.67 |
|
Colouration |
1.21 |
1.25 |
|
Survival |
1.48 |
1.92 |
DISCUSSION
For
the past three decades, the Niger Delta mangrove wetland had consistently has
been subjected to ecological abuse owing primarily to crude oil exploration and
exploitation activity. Indeed uncontrolled exploitation of natural resources in
the eco-region has resulted in declining habitat quality and biodiversity loss.
The mangrove ecosystem is ecologically very sensitive to human perturbation and
natural reestablishment processes have been exceedingly slow. This is reflected
both in the poor rejuventation potential of the
natural vegetation and the effects of contamination from crude oil spill. The
rehabilitation of crude oil impacted habitats will require replanting
strategies and a considerable understanding of the factor associated with the
growth processes, in addition to seedling survival under the prevailing
degraded environmental conditions in the region.
Our
study on the effects of crude oil exposed to different treatments (acute and
chronic) indicated considerable variation in seedling reaction ranging from
growth responses such as stem height, stem girth, leaf length, leaf width,
yellowing of leaves (colouration), leaf loss
(senescence), leaf production and seedling survival.
Our
findings from these experiments on mangrove seedlings exposed to different
crude oil treatments demonstrated hampered growth with respect to the stem
growth -height and girth, leave development including leaf length, width and
yellowing of leaves against the control (that demonstrated greater development
for stem growth -height and girth, leaves and survival of seedlings) in spite
of the non statistical significant difference
observed between various treatments and control. This situation is attributed
to the stringent polycyclic aromatic components
associated with crude oil. This scenario was also demonstrated for mangrove
seedlings under chronic exposure. For instance the development of stem (height
and girth) and leaf (length and width) based on the relative growth rate
suggests that the acute exposure of seedling had more damaging effect on
seedlings than the chronic exposure. Similar observation was made on mangrove
seedlings by Proffitt et.al. (1995), at
different exposure levels (acute and chronic) and demonstrated linear growth
but was less than that of the control.
The
observed differences in response of seedling attributes exposed to the
different treatments with acute having a declining response pattern of stem
height - RGR = 0.17 > stem diameter - RGR = 0.01 > leaf length - RGR = 0
= leaf width - RGR = 0 and chronic with leaf length - RGR = 0.20 > stem
height - RGR = 0.19 > leaf width - RGR = 0.15 > stem diameter - RGR =
-0.03 were further corroborated by cluster and correspondence analysis. These
suggest that mangrove seedling respond differently to crude oil exposure.
Similar studies have indicated such adverse consequences of the negative crude
oil effect on mangrove seedling (Proffitt et al. 1995, DeLaune
et al. 1979, DeLaune
et al. 1990, Duarte et al., 1998). This response trend
provide veritable and important tool for considering effect of crude oil on
mangroves.
The
observed difference between the treated seedling (acute) and control indicates
evidence of negative role of crude oil on mangrove seedling development. This
observed retardation in seedling development particularly on stem height, stem
girth, and yellowing of leaf (chlorosis) with over
50% reduction in growth against the control is relatively in support of similar
studies on the deleterious effect of crude oil on plant development (Baker,
1981a,b; Duarte et al 1998). The decline in leaf width is evidence adduced to
the effect of acute treatment on the seedling. Generally mangrove seedlings
exposed to chronic faired better than the acute
against the control treatment.
The
crude oil level may have also altered the sediment quality (attributes)
firstly; crude oil in the soil may reduce sediment porosity and gaseous
exchange that in turn may have a negative effect on the physiological function
of the plant (Amadi et al. 1997, IPS 1989). Also other possible effect may be hinged on
one of the characteristic of soils polluted by crude oil (petroleum
hydrocarbons) contributing to their low mineral-nitrogen content. This is based on the fact that in the immobilisation of mineral-nitrogen by soil micro-organisms
during the process of degrading the polluting crude oil (petroleum
hydrocarbons). Reduction in mineral-nitrogen contents after oil pollution as a
result of microbial immobilisation has been reported
(Odu, 1972).
Oil pollution adversely affects the availability of mineral nitrogen by
encouraging the rapid growth of soil micro-organisms which immobilise
soil mineral nitrogen and this may be responsible for the yellowing of leaves
observed.
Secondly,
petroleum hydrocarbons induce stress in salt-extracting plants such as the red
Mangroves, by disrupting the ability of the roots to exclude ions from sea or
brackish waters (Page et al, 1985).
Oil stress in salt-excluding halophytes, such as Mangroves, results from
interference by hydrocarbons in this process (Scholander,
1968). Chloride ion exclusion in the
roots of Mangrove seedlings is disrupted by exposure to diesel fuel, and
toluene (Teas, 1979).
In
effect oil stress in Mangroves is an artificially induced hypersalinity
syndrome in which the oil-exposed trees are less able to exclude salt from
their root tissues. Thus sodium, the
principal seawater cation, would be elevated in the
tissues of Mangrove plants unable to exclude salt efficiently in their
roots. Potassium ion, a major physiological
cation serves as a reference. In a healthy tree, the ratio of sodium to
potassium would be smaller than in a tree unable to exclude salt effectively.
Non the less, other
studies on crop plants have indicated similar negative growth pattern on plant
survival and biomass production. Merkl et al.,
(2005) observed death of leguminous plants and reduced biomass production of
grasses exposed to oil contaminated soil. Adoki and Orugbani (2007) observed that non-nutrient supplemented oil
polluted soil recorded low percentage germination; contrary to, contaminated
soil treated with fertilizer supplement that demonstrated enhanced percent
germination. Similarly, reduction in crop yield, declined land productivity and
depressed farm income in oil spill farmland in Delta State of Nigeria had been
observed (Inoni et al.; 2006).
These scenarios suggest that
crude oil have negative consequences both on mangrove plants and agricultural
crops.
ACKNOWLEDGEMENTS
We are indebted to Ifiesimama Oluka, Hanson Uyi, and Nathan Nario for their
kind assistance and advice while we carried out these experiments at the
Institute of Pollution Studies Laboratory, Rivers State University of Science
and Technology, Port Harcourt. We are
indeed grateful to Udonna Ikoro,
the chief laboratory technologist of the Institute of Pollution Studies for
invaluable advice and the use of equipment and facilities for analysis of
samples. More thanks are also due to the eight unanimous reviewers for the
helpful comments and suggestions on the manuscript.
LITERATURE CITED
Amadi, A.; S. D. Abbey and A. Nma. 1996. Chronic effect of oil spill on soil properties
and microflora of a rainforest ecosystem in Nigeria.
Water, Air and Soil Pollutions 86:1-11.
Adoki, A. and T. Orugbani. 2007. Influence of nitrogenous fertilizer plant
effluents on growth of selected farm crops in soils polluted with crude
petroleum hydrocarbons. African Journal of Agricultural Research Vol. 2 (11):
569-573.
Baker, J. M. 1981a. Impact of the petroleum industry on the mangrove
ecology. In: Proceedings of the
Seminar on the Petroleum Industry and the Nigerian Environment. NNPC/FMW &
H, Petroleum Training Institute, Warri, Nigeria, p.71-89.
Baker, J. M. 1981b. Investigation of oil industry influences on tropical
marine ecosystems. Marine Pollution Bulletin 12 (1): 6-10.
Bray, R. T. and J. T. Curtis. 1957. An ordination of the upland forest
communities of Southern Wisconsin. Ecol. Monogr., 27
(4): 325-349.
Choudhry, Junaid
K. 1997. Sustainable management of coastal mangrove development and social
needs. The World Forestry Congress. Antalya, Turkey, 13-22 October, 1993. Vol.
6 - topic 37.
Clarke K. R. and R. N. Gorley. 2001. PRIMER v5: User
manual/tutorial, PRIMER-E, Plymouth UK, 91 pp.
Clarke K. R. and R. N. Gorley. 2006. PRIMER v6: User
manual/tutorial, PRIMER-E, Plymouth UK, 192 pp.
DeLaune, R. D.; W. H. Patrick, Jr.
and R. J. Buresh 1979. Effect of crude oil on a
Louisiana Spartina alterniflora
salt marsh. Environmental Pollution 20: 21-31.
DeLaune, R. D.; R. P. Gambrell, J. H. Pardue and W. H.
Patrick, Jr. 1990. Fate and effect of petroleum hydrocarbons and toxic organics
in Louisiana coastal environments. Estuaries 13 (1): 72-80.
Duarte, C. M.; O. Geertz-Hansen, U. Thampanya,
J. Terrados, M. D. Fortes, L. Kamp-Nielsen, J. Borum and S. Boromthanarath.
1998. Relationship between sediment conditions and mangrove Rhizophora apiculata seedling growth and nutrient
status. Mar. Ecol. Prog.
Ser. 175: 277-283.
Ekweozor, I. K. E.
Ekweozor, I. K. E.
Gobo, A. E. 1988. Relationship between rainfall trends and flooding in
the Niger Delta – Benue River Basin. J. Meterology 13
(132): 220-224.
Imevbore, A. M. A. 1979. The impact of
oil pollution on the biota of the Niger Delta. Seminar on the Environmental
Aspects of Oil Pollution in the Niger Delta, Port Harcourt, Nigeria.
Imevbore, A. M. A. and S. A. Adeyemi. 1981. Environmental monitoring in relation to
prevention and control of oil pollution. In:
Proceedings of the Petroleum Industry and the Nigerian Environment. NNPC/FMOW
& H Warri, Nigeria. p. 135 –142.
International Petroleum Industry Environmental Conservation Association
(IPIECA). 1993. Biological Impacts of Oil Pollution: Mangroves. IPIECA Report
Series. Volume 4. 24 pp.
Institute of Pollution Studies (IPS). 1989. Environmental Data
Acquisition of some NNPC Operational Area . RSUST/IPS/TR/89/03.
Inoni, O. E.; .D. G. Omotor and F. N. Adun. 2006. The
effect of oil spillage on crop yield and farm income in Delta State, Nigeria.
Central European journal of Agriculture 7 (1):41-48.
Keay, R. W. J.; C. F. A. Onochie and D. P. Standfled.
1964. Nigerian Tree Vol.1 and 2. Federal Department of Forestry Research, Ibadan,
Nigeria National Press Ltd.
Monaghan, P. H. and C. B. Koons. 1975. Research needed
to determine the chronic effect of oil on the marine environment. Marine Pollution Bulletin 6 (10):
157-159.
Merkl,
N.; R. Schultze-Kraft and C. Infante. 2005. Assessment of tropical grasses and
legumes for phytoremediation of petroleum-contaminated soils. Water, Air and
Soil Pollution 165 (1/4): 195-209.
Niger Delta Development Commission (NDDC). 2004. Biodiversity of the
Niger Delta environment Niger Delta Development Commission Master Plan Project
Final report.
Nigerian National Petroleum Corporation (NNPC). 1985. Petroleum
exploration and development in Nigeria. Public Affairs Department, NNPC, Lagos,
Nigeria, 14 pp.
Niger Delta Environmental Survey (NDES). 1996. Preliminary Report, 1st
Phase, Vol. 1: 1-96.
Niger Delta Environmental Survey (NDES). 2000. Ecological zonation and habitat
classification. 2nd Phase
Report 2, Vol.1: 1-66.
Norman, R. S.; P. Moeller, T. J. McDonald, and P. J. Morris. 2004.
Effect of Pyocyanin on a Crude-Oil-Degrading
Microbial Community. Applied and
Environmental Microbiology, 70 (7): 4004-4011
Nnyong, E. E. and E. E. Anita.
Odu, C. T. I. 1972. Microbiology
of soils contaminated with petroleum. Hydrocarbon extent of contamination and some
soil and microbial properties affected after contamination. Journal of the
Institute of Petroleum (London) 58: 201-208.
Odu C. T. I., Esuruoso O. F., Nwoboshi L. C.
and Ogunwale J. A. 1985. Environmental Study of the
Nigerian. Agip Oil Company, Operational Area. Soil
and Fresh Water Vegetation Union Graft Publs. Milan
pp.21 – 25.
Page, D. S.; E. S. Giliffan, J. C. Foster, J.
R. Hotham and L. Gonzalez. 1985. Mangrove leaf tissue
sodium and potassium ion concentrations as sublethal
indicators of oil stress in mangrove trees. Proceedings of the 1985 Oil Spill
Conference. American Petroleum Institute, Washington, D.C. p. 391-393.
Proffitt, C. E.; D. J. Devlin and M.
Lindsay. 1995. Effects of oil on mangrove seedlings grown under different
environmental conditions. Marine Pollution Bulletin 30 (12): 788-793.
Research Planning Institute (RPI). 1985. Environmental baseline studies
for establishment of control criteria and standards against petroleum related
pollution in Nigeria. RPI/84/4/15-7.
Scholander, P. F. 1968. How mangroves
desalinate water. Physiologia Plantarum
21: 251-261.
Sneath, P. H. A. and R. R. Sokal. 1973. Numerical taxonomy: the principles and
practice of numerical classification. San Francisco: Freeman, 1973. 573 pp.
Statistical Analysis System (SAS). 2005. JMP version 6.0. SAS Institute Inc., SAS Campus Drive,
Cary, North Carolina 27513. USA.
Snowden, R. J. and I. K. E. Ekweozor. 1987.
The impact of a minor spillage in the Estuarine Niger Delta. Marine Pollution
Bulletin 18 (11): 595-599.
Teas, H. J. 1979. Silviculture with saline water. In
The Biosaline Concept, A. Hollaender
Ed. Plenum Publishing Co., New York, pp 117-161.
Página diseñada por Prof. Jesús Rafael Méndez Natera
TABLA DE CONTENIDO DE LA REVISTA CIENTÍFICA UDO
AGRÍCOLA



