Revista Científica UDO
Agrícola Volumen 12. Número 1. Año 2011. Páginas: 142-154
Bioresource management for improvement of soil chemical and biochemical
quality in arid environment
Manejo de
los biorecursos para el mejoramiento de la calidad química y biológica de los
suelos en ambientes áridos
Ghanshyam TRIPATHI ![]() , R. DEORA and B. M. SHARMA
, R. DEORA and B. M. SHARMA
Department of Zoology, Jai
Narain Vyas University, Jodhpur- 342 001, India. E-mail: drgst@rediffmail.com ![]() Corresponding author
Corresponding author
|
Received: 12/10/2009 |
First reviewing ending: 09/13/2010 |
First review received: 11/01/2010 |
Accepted: 01/05/2011 |
ABSTRACT
Fauna-induced litter decomposition and associated
changes in soil organic carbon (SOC), total soil nitrogen (TSN), soil ammonium
nitrogen (SAN), soil nitrate nitrogen (SNN) and soil available phosphorous
(SAP), soil respiration (SR) and soil dehydrogenase activity (SDA) were studied
in Tecomella undulata (T) tree based
silvipature system integrated with Cenchrus
ciliaris (CC) and Lesiurus sindicus
(LS) grasses in dry region of India. The litter bag experiment was performed
using tree and grass litters. The faunal association was maximum in T+LS
litter. Whereas the litter decomposition was maximum in T + CC litter. Thus
decomposition was influenced by litter mixtures and associated soil fauna.
Faunal population and litter decomposition were highest inside the canopy of
tree at 5 cm depth defining preferred faunal niche. SOC, TSN, SNN, SR and SDA
were significantly (P < 0.05) higher in the mixture of tree and grass
litters than tree litter alone at all decomposition periods. TSN, SAN, SNN,
SAP, SR and SDA were significantly (P < 0.05) higher under the canopy zone.
The higher nutrient enrichment and biochemical activities in the mixture of
litters under the tree canopy at 5 cm depth may be due to the mixing and
decomposition of greater volume of litters by soil biota. However, SOC was significantly (P < 0.05)
higher at surface and minimum at 5 cm depth. It may be due to the loss of
carbon as CO2 by higher microbial population at 5 cm. A positive and significant correlation and
interaction among litter-associated soil fauna, litter decomposition, soil
chemical and biochemical properties clearly demonstrate the importance of soil
fauna in organic resource management in dry areas.
Key words: Dry region, soil fauna, litter decomposition, soil
nutrients, silvipasture system.
RESUMEN
Se estudiaron la descomposición
inducida por la fauna de la hojarasca y los cambios asociados en el carbono
orgánico del suelo (COS), nitrógeno total del suelo (NTS), nitrógeno amónico
del suelo (NAS), nitrógeno en forma de nitrato del suelo (NNS), fósforo
disponible del suelo (FDS), respiración del suelo (RS) y la actividad de la
deshidrogenasa del suelo (ADS) en árboldes de Tecomella undulate (T)
basado en un sistema silvipasture
integrado con las gramíneas Cenchurus
cilliaris (CC) y Lesiurus sindicus
(LS) en la región seca de la India. El experimento en bolsas con hojarasca se
realizó usando hojarascas de arboles y gramíneas. La asociación faunística fue
máxima en T + LS, mientras la descomposición de la hojarasca fue máxima en T +
CC. Así, la descomposición estuvo influenciada por la calidad de la hojarasca y
asociada con la fauna del suelo. La población de la fauna y la descomposición
de la hojarasca fueron mayors dentro del dosel del árbol a 5 cm de profundidad
definiendo el nicho preferido de la fauna. COS, NTS, NNS, RS y ADS fueron
significativamente (P <0,05) mayores en la mezcla de la hojarasca de arboles y gramíneas que en solo hojarasca
de árbole en todos los periodos de descomposición. NTS, NAS, NNS, FDSRS y ADS
fueron significativamente (P <0,05) mayors bajo la zona del dosel. El mayor
enriquecimiento de nutrimentos y
actividades bioquímicas en la mezcla de hojarascas bajo el dosel de los árboles
a 5 cm de profundidad puede deberse a la mezcla y descomposición de volumenes
mayors de hojarascas por la biota del suelo. Sin embargo, COS fue
significativamente (P<0,05) mayor en la superficie y mínimo a 5 cm de
profundidad, lo que puede deberse a la pérdida de carbon en la forma de CO2
por existir una mayor población microbiana a los 5 cm. La correlación positiva
y significativa y la interacción entre la fauna del suelo asociada con la
hojarasca, la descomposición de la hojarasca y las propiedades químicas y
bioquímicas del suelo claramente demostraron la importancia de la fauna del
suelo en el manejo de recursos orgánicos en áreas secas.
Palabras clave: Región árida, fauna del suelo, descomposición de la hojarasca,
nutrimentos del suelo, sistema silvopastoril.
INTRODUCTION
Soil animals impart in several
ecosystem functions such as regulation of nutrient mineralization, litter
decomposition and providing buffer energy source for both plant and soil. Biological function such as the maintenance
of soil fertility is based on the action of organisms including belowground
micro- and macrofauna. One of the major activities occurring in the
pedoecosystem is decomposition. Decomposition is central to the normal
functioning of an ecosystem and it has been estimated that 80-90% of net
primary production in terrestrial ecosystems is recycled by decomposers (Giller
et al., 1997). Since a great
proportion of the nutrients in tropical ecosystems are incorporated into the
organic matter, the decomposition is an important process for regenerating the
nutrients to support production in the ecosystem (Cuevas and Medina, 1986). It
provides basic clues in understanding and estimating productivity, energy-flow
and nutrient cycling (Johansson, 1994).
Soil cannot perform ecosystem
services like decomposition, nutrient cycling and disease suppression without
an array of soil organisms. Soil fauna affect primary
production directly by root-feeding and indirectly through their contribution
to decomposition and nutrient mineralization (Crossley et al., 1992). Microbial-grazing mesofauna affect growth and
metabolic activities of microbes and alter community composition, thus
regulating decomposition rate of organic matter (Yeates and Coleman, 1982;
Seastedt, 1984). Increasing rates of litter decomposition accelerate nutrient
cycling within the site and indicate increased soil quality (Knoepp et al., 2000).
Publications of reports on sustainable land
use and soil biodiversity by various international organizations leave the
impression that soil fertility is controlled by soil biodiversity. It means that low soil fertility occurs
together with a decrease in soil biodiversity.
This is the point of attraction for biologists to look into the role of
below-ground faunal biodiversity in maintaining sustainability of soil system.
Since valuable reports propagate below-ground biodiversity as a soil health
indicator, we take an opportunity here to critically and experimentally analyze
this notion. Therefore, inventory and conservation of soil bioresources must be
a priority. A diverse, balanced and active soil biota could help provide soil
conditions necessary for sustainable land production through increased
microbial activity, carbon turnover and nutrient supply, preventing plant pathogens,
supporting the populations of beneficial organisms, reducing loss of inorganic
fertilizers through erosion and leaching by short-term immobilization,
stabilizing soil structure, and reducing reliance upon hazardous agrochemicals.
Several properties or functions of soil fauna can be
used to indicate soil health (Gupta and David, 2005).
The Indian arid silvipasture land is
characterized by harsh climatic conditions including low and erratic rainfall,
high air temperature and intense solar radiation coupled with high wind
velocity and nutrient deficiency. Recurring drought and famines are common
features in the region. An efficient and
judicious management of silvipasture resources is very essential due to poor
soil quality in dry region. Selection of an appropriate combination of tree and
subvegetation (grass, crop) and development of suitable management practices
like litter decomposition, pruning, lopping and thinning are important aspects.
There is very little information on relationship between soil faunal
resources and sustainability of agroforestry systems. So it appears necessary
to evaluate the role of soil invertebrates in litter decomposition and nutrient
enrichment. Litter resource management may optimize the below-ground biological
activities for sustainable land use in arid environment. Tecomella undulata is a common tree of traditional agroforestry
system of the northwestern dry region of India and belongs to the family
Bignoniaceae. Unfortunately, this species has become victim of overexploitation
for its high quality timber and medicinal values (Poffenberger et al., 1992) and is now listed in the
category of endangered species in the Thar desert (Khan, 1997). Therefore,
fauna associated litter decomposition, nutrient dynamics and biochemical
changes were studied in T. undulata
based silvipasture system of dry region.
MATERIALS
AND METHODS
Site description
Studies were conducted in
Jodhpur district of Rajasthan in India.
It is situated between 260 45` north latitude and 720
03` east longitude in arid region. The
climate of the region is dry tropical type characterized by extremes of
temperature, fitful and uncertain rainfall, high potential evapotranspiration and strong winds. Three prominent
seasons in the year are summer, monsoon and winter. Summer is the most dominant season
characterized by high temperature spreading from March to middle of July. The period from mid July to September is the
monsoon season, when most of the rainfall is received. The winter season spreads from November to
February. The most important characteristic feature of the arid climate is the
wide variations in diurnal and temporal temperature.
Experimental
procedures
The leaf litter of T. undulata (T) and grass litters of Cenchrus ciliaris (CC) and Lesiurus
sindicus (LS) were harvested, chopped and allowed to dry. A particular
amount of tree litter alone and along with grasses (CC, LS) were kept in a
nylon bag of 7 mm mesh size. These litter bags were placed on horizontal and
vertical positions in six replications in four hectare area of T. undulata tree plantation to study the
quantification and kinetics of fauna-associated decomposition. Horizontally,
they were placed outside and inside the canopy of tree. Vertically, the litter
samples were placed on surface, 5cm and 10cm depth. Bags were taken out from
each position at an interval of four months. The fauna-associated with litter
decomposition were extracted by Tullgren funnel, identified and counted
(Crossley and Coleman, 1999).
Decomposition associated
changes in chemical and biochemical properties of soil such as soil organic
carbon (SOC), total soil nitrogen (TSN), soil ammonium nitrogen (SAN), soil nitrate nitrogen (SNN), soil
available phosphorous (SAP), soil respiration (SR) and soil dehydrogenase
activity (SDA) were analyzed as described by Anderson and Ingram (1993). Soil organic carbon was determined by Walkley
and Blacks wet- digestion method (Walkley and Black, 1934). Total nitrogen was
estimated by Kjeldahl method (Bremner, 1960). Ammonium nitrogen, nitrate nitrogen (Mulvaney, 1996) and
available phosphorous (Olsen y Sommers, 1982) were measured
spectrophotometrically. Soil respiration (SR) and soil dehydrogenase activity
(SDA) were determined using potassium hydroxide (Franzluebbers et al., 1995)
and triphenyl tetrazolium chloride (Casida et al., 1964), respectively.
The data recorded from
different experiments on decomposition, nutrient dynamics and biochemical
changes associated with faunal population were analyzed statistically. Since
all the observations for the same study site were available for different time
intervals, the data was studied by repeated-measure
design to test the level of significance. Duncan’s Multiple Range Test (DMRT)
was performed for the entire analysis to obtain homogenous subsets among the
litter qualities and soil depths. Pearson correlation coefficient was
calculated to know the relationship between the faunal population and litter
decomposition, soil chemical and biochemical properties. The level of
significance was set at 0.05.
RESULTS
AND DISCUSSION
Quantification
and kinetics of fauna associated with litter decomposition
The faunal population
association was significantly (P < 0.003) higher in T+LS litter. However,
litter decomposition was significantly (P < 0.001) greater with T + CC
(Table 1 and Figure 1). The mixture of two species litters generally decomposed
faster than a single one. Increased decomposition and higher density of fauna
in mixed litter may be due to diverse chemical composition attracting a variety
soil fauna. Probably the abundance and activity of invertebrates was influenced
by the initial litter chemistry (Zimmer, 2002). Schadler and Brandl (2005)
described that different species of invertebrates may be attracted to certain
litter types and with an increasing richness of litter decomposer may show
complementary resources use, thereby higher faunal population associated with
the mixture of litters. An increased number of
trophic levels would increase decomposition rate (Bengtsson et al., 1995).
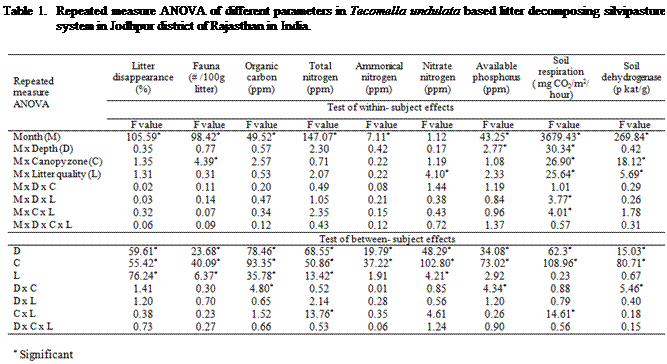
While considering the mean of
all variables for canopy zone, faunal association and litter disappearance was
significantly higher inside the canopy of T.
undulata as compared to outside. Depth-wise
variation of the faunal population and litter decomposition was significantly
(P < 0.001) greater at 5 cm. whereas it was lowest at surface layer. This
shows that soil fauna associated with litter decomposition preferred niche
inside the tree canopy and at 5 cm depth. Faunal population and litter
disappearance varied significantly (P < 0.001) due to changes in months.
Both faunal association and litter disappearance were higher over the first
four months of decomposition and it was reduced as a function of time interval.
Due to sufficient availability of litter as food and best climatic condition of
rainy season for growth and development of soil fauna, the disappearance of
litter and associated fauna were higher over the first four months of decomposition. The percentage of decay increased with the
increasing amounts of rainfall and humidity.
The highest decomposition of
organic matter was observed under conditions of moderate temperature (30°C) and
soil moisture content (60-80%) (Kononova, 1975). Nearly similar climatic
condition was found during first four months of litter decomposition. Shanks and Olson (1961) compared litter decay beneath
natural stands at various elevations and concluded that there was an average
decrease in breakdown of nearly 2 percent for each 1°C drop in mean
temperature. Lang (1974) found five folds higher decay of litter during autumn
as compared to the winter and summer. Boonyawat and Ngampongsai (1974) also
observed the highest decomposition of evergreen forest litter in the late rainy
season and early winter and the lowest rate in summer. Brinson (1977)
and Vander Drift (1983) pointed out that
precipitation and temperature were important factors for litter decomposition
because they affect both the development of plant cover and the activities of
soil fauna, which are highly critical factors in litter decompostion.
The amount of litter decomposition and faunal population decreased
after a span of months. Schimel and Gulledge (1998) suggested that the
corresponding decrease in litter decomposition and faunal population may be due
to the changes in soil and litter moisture. The consequences of climate change
are likely to induce changes within functional groups or shifts in the balance
between different functional groups in the soil decomposer community, which
could significantly affect litter decomposition (Swift et al., 1998). The test of
within-subject effects of month x canopy interaction was highly significant (P
< 0.05) for litter fauna. Litter decomposition and associated fauna showed a
significant positive correlation (P< 0.05) during all decomposition periods
(Table 2). A positive and significant (P< 0.05) interaction and correlation
between litter associated soil fauna and litter decomposition clearly demonstrated
the positive impact of soil fauna on litter decomposing activities in
silvipasture system of desert region. It was observed that the litter
decomposition varied as a function of associated fauna in different litters.
This proves that decomposition was influenced by litter quality and associated
soil fauna.
|
Table 2. Correlation of
soil faunal population with litter loss, organic carbon, total nitrogen
and ammonium nitrogen,
nitrate nitrogen, available phosphorus, soil respiration and soil dehydrogenase
activity in Tecomella undulata based
silvipasture system at different time intervals in Jodhpur district of Rajasthan in India. |
|||||||
|
Parameter |
Decomopostion period
(months) |
||||||
|
4 (Oct.) |
8 (Feb.) |
I2 (June) |
|||||
|
r- Value |
P-Value |
r- Value |
P-Value |
r- Value |
P-Value |
||
|
Litter loss |
0.575 |
< 0.001 |
0.524 |
< 0.001 |
0.470 |
< 0.001 |
|
|
Organic carbon |
0.125 |
ns |
0.015 |
ns |
0.103 |
ns |
|
|
Total nitrogen |
0.389 |
< 0.001 |
0.273 |
< 0.004 |
0.382 |
< 0.001 |
|
|
Ammonium
nitrogen |
0.372 |
< 0.001 |
0.289 |
< 0.002 |
0.295 |
< 0.002 |
|
|
Nitrate nitrogen |
0424 |
< 0.001 |
0.286 |
< 0.001 |
0.348 |
< 0.001 |
|
|
Available phosphorus |
0.324 |
< 0.001 |
0.436 |
< 0.001 |
0.426 |
< 0.001 |
|
|
Soil respiration |
0.527 |
< 0.001 |
0.375 |
< 0.001 |
0.398 |
< 0.001 |
|
|
Soil dehydrogenase activity |
0.486 |
< 0.001 |
0.527 |
< 0.001 |
0.336 |
< 0.001 |
|
|
Sampling months are in
bracket; ns, Nonsignificant |
|||||||
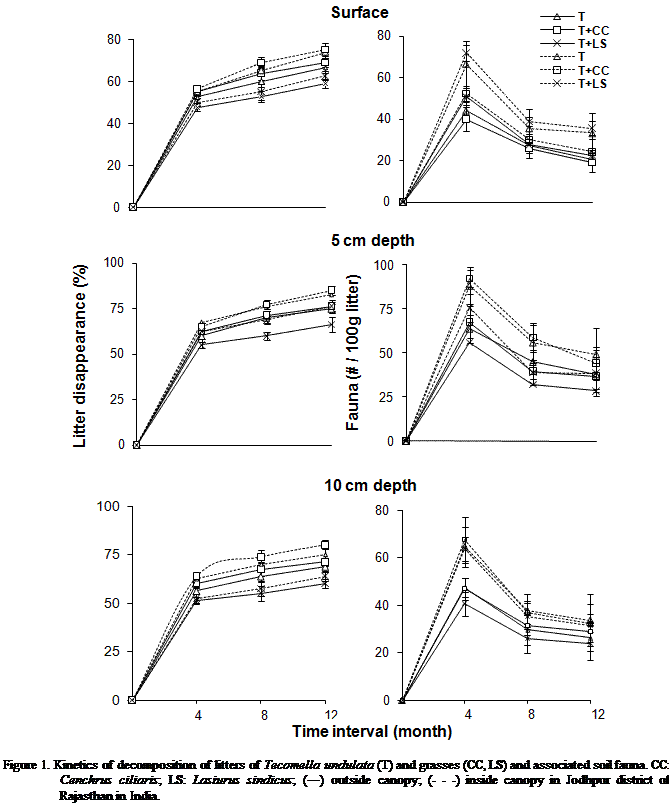
Decomposition dependent chemical changes
SOC, TSN and SNN varied
significantly (P < 0.001) due to changes in litter quality. Concentrations
of these nutrients were greater in T + LS litter. Considering the mean of all
variable for canopy zone, SOC, TSN, SAN, SNN and SAP were significantly (P <
0.001) higher inside the canopy as compared to outside the canopy of T. undulata (Figures 2, 3 and 4).
Depth-wise variations in TSN, SAN, SNN and SAP were significantly (P <
0.001) greater at 5 cm depth. Whereas they were lowest at surface. The nutrient
enrichment of the soil under tree canopy was due to mixing and decomposition of
greater volume of litters through soil biota. Further the nearest zone would
have received more nutrients from the tree since the soil adjacent to the tree
trunk had been covered by the canopy for the longest period which supports the
establishment of decomposer community for higher decomposition. However, SOC
was significantly (P < 0.000) greater in top soil layer and lowest at 5 cm depth.
It may be due to the loss of carbon as CO2 by higher microbial
population at 5 cm. About 60% of the carbon in organic materials are respired
as carbon dioxide and 40% is retained as bacterial biomass (Ingham, 2007).
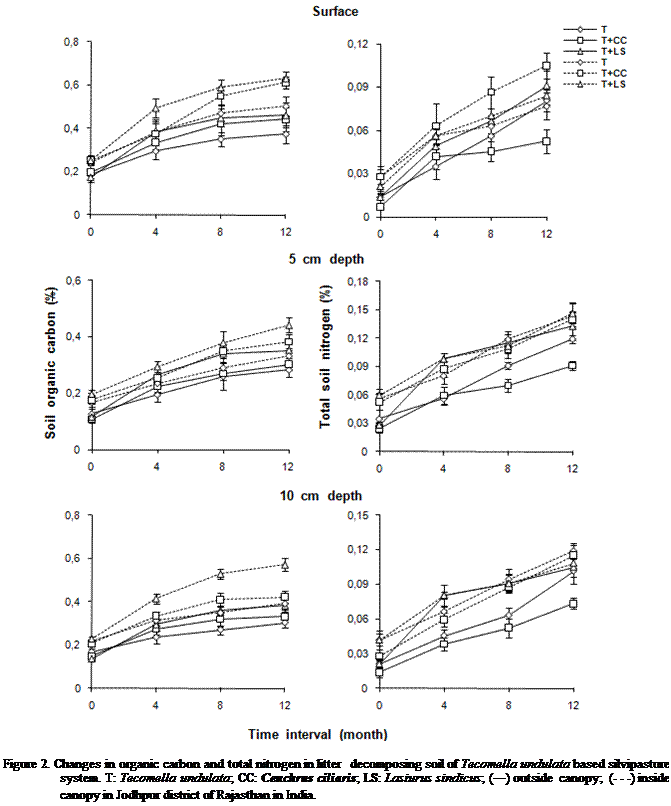
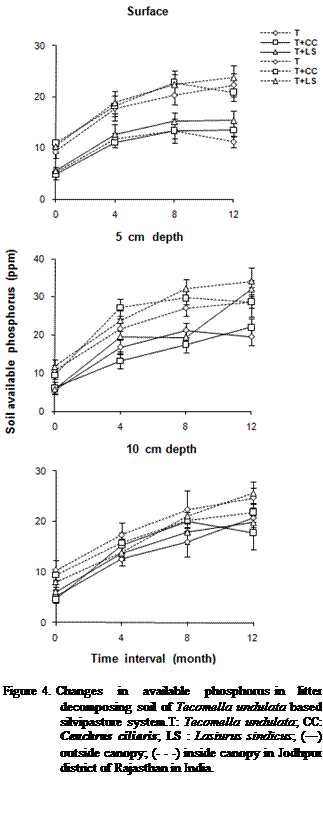
SOC, TSN, SAN, SNN and SAP changed significantly (P < 0.000) due to
changes in months. About 2 to 4 fold higher nutrient concentrations were
obtained after twelve month of decomposition as compared to initial values. Over
the first four months of decomposition the increments in the soil nutrients
were higher than the other periods of decomposition. The increase in nutrients
except soil organic carbon at depth may be due to leaching and deposition of
elements. Muoghalu and Awokunle (1994) studied the spatial pattern of soil
properties under tree canopy in a forest region and reported a significant
decrease in organic matter with soil depth and distance from the tree base.
They also showed a significant decrease in soil nitrogen and significant
changes in phosphorus content with the distance from tree base.
The concentration of soil
organic carbon, total nitrogen, ammonium nitrogen, nitrate nitrogen, available phosphorous were 1.5 –3 fold
higher after the twelve months of decomposition suggesting improvement in
nutrient status. Coleman et al. (1992) documented bacteria and fungi as the major nutrient
cycling processors in soil. The waste products of bacteria produce soil organic
matter and thus increase the level of organic carbon in soil. When microarthropods graze on fungul and bacterial infected
litter, some of the nitrogen bound in these microbes is mineralized and
released as nitrogenous waste, and increase soil nutrient (Whitford et al., 1982). Rao and Tarafdar
(1992) reported vegetation cover, soil temperature and soil moisture as an
important variable for the different status of phosphorus in soil. Organic
matter in soil is the most important fraction that supports microbial
populations. Microbial biomass (MB), the living component of soil organic
matter, constitutes 2-5% of the organic carbon in soils. MB acts as the engine
for organic matter turnover and nutrient release (Ingham, 2007). Therefore,
higher nutrient concentration was obtained at greater faunal density during
decomposition.
Pramanik et al. (2001) studied nutrient mobilization from leaf litter by
detritivore soil arthropods and documented significantly high rates of organic
carbon and nitrate release by soil fauna. It also supports the present findings
of higher nitrogen content at a greater faunal density in litter decomposing
places. Griffiths (1994) estimated from several independent food web studies
that soil microfauna were responsible for 20–40% of net nitrogen mineralization
under field conditions. In addition, leaching from damaged fungal hyphae due to
mesofauna grazing may also increase ammonia content in soil. Beare (1997)
reported that fungal-feeding microarthropods are very important in mobilizing
nutrient from surface residues through grazing. Bacterial-feeding and predatory
soil fauna are estimated to contribute directly and indirectly about 8 to 19%
of nitrogen mineralization.
The test of between-subject effect of depth x canopy zone was
significant for SOC and SAP. While the interaction between canopy and litter
quality was significant (P < 0.000) for TSN. Whereas depth x canopy was
significant (P < 0.000) for SAP. Associated fauna showed a significant
positive correlation (P< 0.05) with TSN, SAN, SNN and SAP during all
decomposition periods (Table 2). A positive and significant correlation and
interaction among litter-associated soil fauna and soil nutrients during
decomposition period clearly demonstrated the impact of fauna on soil
nutrients. The increased rates of nutrient mineralization suggested a more
rapid cycling of organic matter and greater amounts of nutrients availability
by soil fauna-induced litter decomposition. The present observations on soil
arthropod associated changes in nutrient status may be supported by the report
of Maity and Jay (1999) who described that the colonization of microarthropods
have a significant role in trapping energy and nutrients from decomposing
litter and in enhancing biological activity in soil. Kumar et
al. (1999) also found high diversity and density of soil fauna with very
high nutrient status in soil. They
remarked that high fertility and nutrient status of the soil may be due to the
presence of the diverse soil fauna which assist in humus formation. The increase in soil nutrients was associated
with the increase in soil faunal population. It reflected fauna-induced
increase in decomposition activities in soil. The strategy may be adopted for
decomposition of litters and improvement of soil. Therefore, the litter and
fauna management may increase the productivity of T. undulata based silvipasture system on a sustainable basis in dry
areas.
Decomposition dependent biochemical changes
While considering the mean of all variables for canopy zone, SR and SDA
were significantly (P < 0.001) higher inside the canopy as compared to
outside (Figure 5). Depth-wise variation in SR and SDA were significantly (P < 0.001) greater at 5 cm. Whereas
they were lowest at surface. SR and SDA varied significantly (P < 0.001) due
to changes in months. Approximately 3 to 5 fold higher SR and SDA were found at
all position over the first four months of intervals. However, they gradually
decreased as a function of time interval but remained higher after the twelve
months as compared to initial levels. Differences in
soil respiration rates among distant sites may be due to climatic differences
(Raich and Potter, 1995). Other factors which potentially influenced the
rates of soil respiration are the availability of carbon substrate for
microorganisms (Seto and Yanagiya, 1983), soil biota population (Singh and
Shukla, 1977; Rai and Srivastava, 1981), soil physical and chemical properties
(Boudot et al., 1986) and soil
drainage (Luken and Billings, 1985; Moore and Knowles, 1989; Freeman et al., 1993). Tewary et al. (1982) found that soil
respiration rates beneath coniferous trees were lower than those beneath
broad-leaved trees in a mixed forest in Northern India. Dehydrogenases give us
information about the influence of natural environmental conditions of the microbial
activities of the soil because they are more related to the metabolic state of
microbial population. Seasonal variations in the enzymatic activities of
soil are biologically important because they change the quantity and quality of
substrates upon which they act and are responsible for altering the rate of
various soil processes. Soil enzyme activities are
often closely related to soil organic matter, soil physical properties, and
microbial activity and biomass (Tate, 1995).
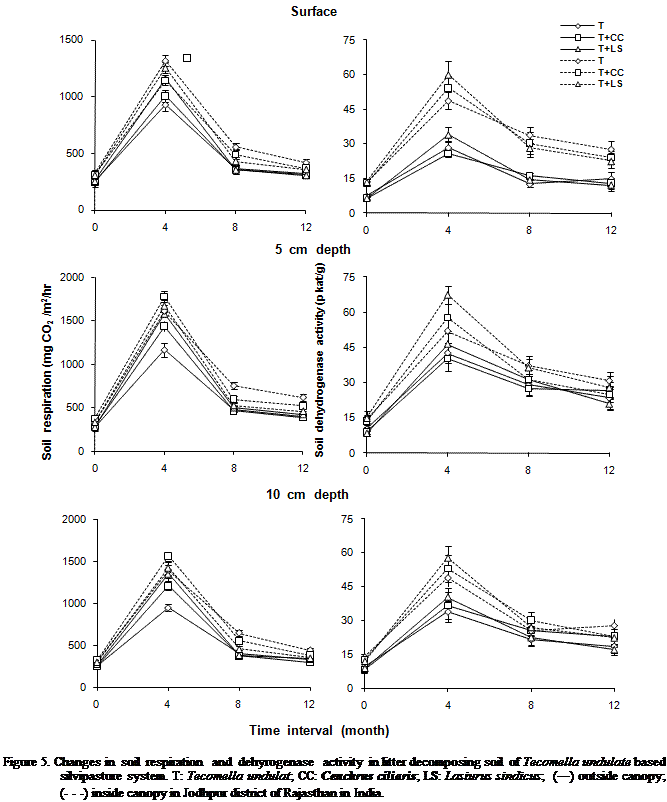
As dehydrogenase activity
reflects the activity microorganisms in the soil (Lenhard 1956), the higher dehydrogenase
activity during the rainy season may be due to optimum moisture and temperature
for the growth of microorganisms at that time (Rao and
Venkateswarlu, 1993). They also observed significantly higher population of
different microorganisms during July-August. Dormaar et al. (1984) observed low activities of dehydrogenase during
summer in mixed prairie of Canada. In many desertic soils, higher temperatures
and soil drying during summer months bring down the microbial population to
very low levels (Sasson, 1972) resulting in low dehydrogenase activities. In
winter low dehydrogenase activity might be due to the fact that the
microorganisms remain in a state of biochemical inactivity (Milosevic 1988).
Therefore, there was a gradual decrease in soil dehydrogenase activity after
four months of rainy season at litter decomposing sites.
The test of within-subject effects of month x canopy, month x litter
quality were significant (P < 0.001) for SR and SDA. Whereas month x
depth, month x depth x litter quality
and month x canopy x litter quality were only significant (P < 0.001) for
SR. While the test of between-subject effect of
canopy x litter quality and depth x canopy interaction was significant
(P < 0.001) for SR and SDA respecively. Associated fauna showed a
significant positive correlation (P < 0.05) with SR and SDA during all
decomposition periods (Table 2). A positive and significant correlation and
interaction among litter-associated soil fauna, soil respiration and
dehydrogenase activity during decomposition period clearly demonstrated the
impact of fauna on biotic activities. The changes in soil respiration and
dehydrogenase activity along with changes in soil faunal population disclosed
the possibility of a strong relationship between soil faunal activity and
functional aspects of soil. The increase
in faunal population with the increase in soil respiration and dehydrogenase
activity clearly reflects the role of soil fauna in improving functional
aspects of soil. High fertility and nutrient status of the soil may be due to
the presence of the diverse soil fauna which may assist in humus formation. A
positive and significant correlation and interaction among litter-associated
soil fauna, litter decomposition, soil chemical and biochemical properties
during decomposition suggested the impact of fauna on soil health
CONCLUSIONS
The colonization of
microarthropods have a significant role in trapping energy and nutrients from
decomposing litter and enhancing biological activity in soil. The present
findings may be useful in restoration and enrichment of degraded level in dry
areas through management of litter resources and soil biota.
ACKNOWLEDGEMENTS
Authors are grateful to Indian Council of Agriculural
Research (ICAR), New Delhi, for provding financial support in the form of a
major research project. BMS and RD are obliged to ICAR for RA and SRF,
respectively. Authors are also thankful to P. S. Pathak, Ex.-Director (IGFRI,
Jhansi) and O. P. Sharma, Ex.-Principal Scientiest (ICAR, New Delhi) for
encouragement and support all the time.
LITERATURED
CITED
Anderson,
J. M. and J. S. I. Ingram. 1993. Tropical soil biology and fertility: A
handbook on methods, CAB International, Wallingford. United Kingdom.
Beare,
M. H.; M. V. Reddy, G. Tian and S. C. Srivastava. 1997 Agricultural intensification,
soil biodiversity and agroecosystem function in the tropics: the role of the
decomposer biota. Applied Soil Ecology 6: 87-108.
Bengtsson, J.; D. W. Zheng, G. I. Agren and
T. Persson. 1995 Food webs in soil: an interface between population and
ecosystem ecology. In: Linking
Species and Ecosystems. C Jones and J. Lawton (eds). Chapman and Hall, New
York, United States of America. p. 159-165.
Boonyawat, S. and C. Ngampongsai. 1974. An
analysis of accumulation and decomposition of litter fall in hill evergreen
forest, Doi Pui, Chiangmai. Kasetsart Univ. Kogma Watershed Res. Bull., 17. 21
p. (In Thai with English summary)
Boudot, J.
P.; B. A. Bel Hadj and T. Chone. 1986. Carbon mineralization in andosols and
aluminium-rich highland soils. Soil Biol. Biochem. 18: 457-461.
Bremner, J.
M. 1060. Determination of nitrogen in soil by the Kjeldahl method. Journal of
Agricultural Science 55 (1): 11-33.
Brinson, M. M. 1977. Decomposition and
nutrient exchange of litter in an alluvial swamp forest. Ecol., 58: 601-609.
Casida Jr, L. E.; D. A. Klein and T.
Santoro. 1964. Soil dehydrogenase activity. Soil Sci. 98: 371-376.
Coleman, D.
C.; E. P. Odum and D. A. Crossley Jr. 1992. Soil biology, soil ecology and
global change, Biology and Fertility of Soils 14: 104-111.
Crossley,
D. A. Jr.; B. R. Mueller and J. C. Perdue. 1992. Biodiversity of
microarthropods in agricultural soils: relations to processes. Agriculture,
Ecosystems and Environment 40: 37-46.
Crossley
Jr.; D. A. and D. C. Coleman. 1999. Microfauna. In: M. E. Sumner (Ed.). Handbook of Soil Science. C-59-C-65. CRC
Press, Boca Raton. United States of America.
Cuevas, E. and
E. Medina. 1986. Nutrient dynamics within Amazonian forests: Part I. Nutrient
flux in fine litter fall and efficiency of nutrient utilization. Oecologia 68: 466-472.
Dormaar, J.
P.; A. Johnston and S. Smoliak. 1984. Seasonal changes in carbon content and
dehydrogenase, phosphatase and urease activities in mixed prairie and fescus
grassland Ah horizon. Journal of Range Management 37: 31-35.
Franzluebbers,
A. J.; R. L. Haney and F. M. Hons. 1995. Soil nitrogen mineralization potential
for improved fertilizer recommendations and decreased nitrate contamination of
ground water. Technical Report No. 171, Texas Water Resources Institute.
Freeman C.;
M. A. Lock and B. Reynolds. 1993. Fluxes of CO2, CH4 and
N2O from a Welsh peatland following simulation of water table
draw-down: Potential feedback to climatic change. Biogeochemistry 19: 51-60.
Gupta,
V. V. S. R. and K. R. David. 2005. Overview - soil biology, nutrient cycling
and disease supression - what can soil biota contribute? Grain Research and
Development. (Online). http://www.grdc.com.au/ Research-and-
Development/GRDC-Update-Papers?pg=3&f=3&t=O
Giller,
K. E.; M. H. Beare, P. Lavelle, A. M. N. Izac and M. J. Swift. 1997.
Agricultural intensification, soil biodiversity and agroecosystem function.
Applied Soil Ecology 6: 3-16.
Griffiths
B. S. 1994. Soil nutrient flow. In:
Soil Protozoa. J. Darbyshire (ed.). CAB International, Wallingford, Oxon,
United Kingdom. p. 65-91.
Ingham, E.
R. 2007. Soil biology primer. (Online). http://soils.usda.gov/sqi/concepts/soil_biology/biology.html.
Johansson,
M. B. 1994. Decomposition rates of Scots pine needle litter related to site
properties litter quality, and climate, and climate. Can. J. For. Res., 24:
1771-1781.
Knoepp, D. J.;
C. D. Coleman, D. A. Crosseley and S. J. Clark. 2000. Biological idiocies of
soil quality: an ecosystem case study of their use. Forest Ecology and
Management 138: 357-368.
Kononova, M. M. 1975. Humus of virgin and
cultivated soils. In: Soil components. J. E. Gieseking
(ed.), Springer-Verlag. New York, United States of America. I: 475-526.
Kumar, M. G.
S.; M. P. Sujatha and S. Shankar. 1999. Population density and diversity of
microarthropods and anneilids in the reed growing. J. Tropical Fort. 15:
135-143.
Lang, G. E. 1974. Litter dynamics in a
mixed oak forest on the New Jersey Piedmont. Bull. Torrey Bot. Club, 101: 277-286.
Lenhard, G.
1956. The dehydrogenase activity in soil as a measure of the activity of soil
microorganisms. Zeitschrift Furpflan- Zeneranaehrange and Bodenkunde 73: 1-11.
Luken, J. O.
and Billings, W. D. 1985. The influence of microtopographic heterogeneity
on carbon dioxide efflux from a subarctic bog. Holarctic Ecol. 8:
306-312.
Maity, S. K.
and V. C. Joy. 1999. Impact of anti-nutritional chemical compounds of leaf
litter on detrivore soil arthropods fauna. J. Ecobiol. 11:
193-202.
Moore, T. R.
and R. Knowles. 1989. The influence of water table levels on methane and
carbon dioxide emissions from peatland soils. Can. J. Soil Sci. 69: 33-38.
Mulvaney, R.
L. 1996. Nitrogen – Inorganic forms. In: Methods of soils analysis. Part 3. D.
L. Sparks et al. (ed.). Soil Sci. Soc. Amer. Book Serie 5. SSSA. Madison,
Wisconsin. USA. p. 1123-1174.
Muoghalu, J.
I. and J. O. Awokunle. 1994. Spatial patterns of soil properties under tree
canopy in Nigerian rain forest region. Tropical Ecology 35: 219-228.
Olsen S. R.
and L. E. Sommers. 1982. Phosphorus. In:
Methods of soil analysis, Part 2, Chemical and microbiological methods. A. L.
Page, D. R. Miller and D. R. Kearney (eds). American Society of Agronomy, SSSA
Madison, Wisconsin, USA. p. 403-430.
Powers, R. F.; A.
E. Tiarks and J. R. Boyle. 1998. Assessing soil quality: practicable standards
for sustainable forest productivity in the united states. In: The Contribution of
Soil Science to the Development of and Implantation of Criteria and Indicators
of Sustainable Forest Management. Soil Sci. Soc.A. Special Pub. Madison,
Wisconsin, United States of America. p. 53-80.
Pramanik, R.;
K. Sarkar and V. C. Joy. 2001. Efficiency of detritivore soil arthropods in
mobilizing nutrients from leaf litter. Tropical Ecology 42 (1): 51-58.
Rai, B. and
A. K. Srivastava. 1981. Studies on microbial population of a tropical dry
deciduous forest soil in relation to soil respiration. Pedobiol. 22: 185-190.
Rao, A. V.
and J. C. Tarafdar. 1992. Seasonal changes in available phosphorus and
different enzyme activities in arid soil. Annals of Arid Zone 31: 185-189.
Rao, A. V. and B. Venkateswarlu. 1993
Microbial ecology of the soils of Indian desert. Agiculture Ecosystem and Environment 10: 361-369.
Russell, E. W.
1969. The soil environment. J.
T. Sheals (ed.). Systematic Association Publ. No. 8.
Sasson, A. 1972. Microbial life in arid environments. Prospects and
achievements. Annals of Arid Zone 11:
67-86.
Schadler,
M. and R. Brandl. 2005. Palatability, decomposition and insect herbivore;
patterns in a successional old-field plant community. Oikos 103: 121-132.
Schimel,
J. P. and J. Gulledge. 1998. Microbial community structure and global trace
gases. Global Change Biology 4: 745-758.
Seastedt, T.
R. 1984. The role of microarthropods in decomposition and mineralization
processes. Annual Review of Entomology 29: 25-46.
Seto, M. and
K. Yanagiya. 1983. Rate of CO2 evolution from soil in relation to
temperature and amount of dissolved organic carbon. Jap. J. Ecol. 33: 199-205.
Shanks, R. E. and J. S. Olson. 1961.
First-year breakdown of leaf litter in southern Appalachian forests Sci. 134:
194-195.
Singh, U. R.
and A. N. Shukla. 1977. Soil respiration in relation to mesofaunal and mycofloral
populations during rapid course of decomposition on the floor of a
tropical dry deciduous forest. Rev. Écol. Biol. Sol. 14: 363-370.
Swift,
M. J.; N. O. Andre and L. Brussaard. 1998. Global change, soil biodiversity,
and nitrogen cycling in terrestrial ecosystems: three case studies. Global
Change Biology 4: 729-743.
Tate,
R. L. 1995. Soil microbiology. Wiley and Sons, New York, United States of America.
Tewary, C.
K.; U. Pandey and J. S. Singh. 1982. Soil and litter respiration rates in
different micro habitats of a mixed oak-conifer forest and their control by
edaphic conditions and substratequality. Plant Soil 65: 233-238.
Walkley, A.
and I. A. Black. 1934. An examination of Degtjareff method for determining soil
organic matter and a proposed modification of the chromic acid titration
method. Soil Sci. 37: 29-37.
Whiteford,
W. G. 1996. The importance of the biodiversity of soil biota in arid ecosystems.
Biodiversity and Conservation 5:
185-195.
Yeates, G.
W. and D. C. Coleman. 1982. Nematodes in decomposition. In:
Nematodes in Soil Ecosystems. D. W. Freckman (Ed.). University of Texas,
Austin. United States of America. p
55-80.
Zimmer, M. and
W. Toppp. 1999. Relationship between woodlice (Isopoda : Oniscidea) and
microbial density and activity in the field. Biology and Fertility of Soils 30: 117-123.
Página
diseñada por Prof. Jesús Rafael Méndez Natera
TABLA DE CONTENIDO DE LA REVISTA
CIENTÍFICA UDO AGRÍCOLA



